* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 6967-module-hfn-206-communicable-dx-1
Survey
Document related concepts
Urinary tract infection wikipedia , lookup
Social history of viruses wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Gastroenteritis wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Neonatal infection wikipedia , lookup
Hepatitis B wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Marburg virus disease wikipedia , lookup
Infection control wikipedia , lookup
Onchocerciasis wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Neglected tropical diseases wikipedia , lookup
Germ theory of disease wikipedia , lookup
Transcript
KENYATTA UNIVERSITY
SCHOOL OF APPLIED HUMAN SCIENCES
DEPARTMENT: FOODS, NUTRITION AND DIETETICS
HFN 206 – COMMUNICABLE DISEASES
WRITTEN BY:
JUSTUS O. S. OSERO
i
INTRODUCTION
I have a great pleasure in presenting the module “communicable diseases”. Communicable
diseases are diseases that are the result of a causative organism spreading from one person to
another or from animals to people.
They are among the major causes of illnesses in Kenya and the entire Africa. These diseases
affect people of all ages but more so children, due to their exposure to environmental
conditions that support the spread. Communicable diseases are preventable if interventions
are placed at the various levels of transmission of the disease.
In recent years our region also faces new and emerging diseases which are challenging public
health as never before. Unfortunately, many of these diseases affect the poor and marginalized
sections of society, and contribute not only to ill health and poverty at micro-level but also
have serious socio-economic implications at the macro-level. Health workers including
Nutritionist have an important role to play in the control of these diseases by applying
effective and efficient management, prevention and control measures. As a health worker you
need to be equipped with the capacity to target communicable diseases for their eradication.
It is hoped that this module will offer the students of Foods, Nutrition, and Dietetics enough
exposure to the subject matter and enable them in their success. I am sure that students will
come out with flying colours if they strictly and sincerely make use this module
It is a sense of honour and pride for me to place on record my sincere thanks and deep sense
of gratitude to well-wishers and guide Professor J. Kimiywe and Head of Department of
Foods, Nutrition, and Dietetics Kenyatta University.
ii
MODULE OBJECTIVES
By the end of this course, students will be able to:
Describe the concepts and classification of communicable diseases.
Describe disease causing organisms.
Discuss common tropical diseases in terms of distribution, etiology, risk
factors, signs and symptoms, diagnosis, treatment and, prevention and
control.
Describe various emerging and re-emerging diseases.
Explain sexually transmitted diseases including HIV and AIDS
Gender aspects of communicable diseases.
iii
TABLE OF CONTENTS
INTRODUCTION ....................................................................................................................................ii
MODULE OBJECTIVES .......................................................................................................................iii
TABLE OF CONTENTS ........................................................................................................................ iv
LECTURE ONE ...................................................................................................................................... 1
CONCEPTS, DEFINITIONS AND CLASSIFICATION ....................................................................... 1
OF COMMUNICABLE DISEASES ...................................................................................................... 1
1.1 Introduction ................................................................................................................................... 1
1.2 Objectives ...................................................................................................................................... 1
1.3 Concepts of Communicable Diseases ........................................................................................... 1
1.3.1 Meaning of communicable diseases ....................................................................................... 1
1.3.2 Common Characteristics of Communicable Diseases ............................................................ 2
1.3.3 Communicable disease cycle .................................................................................................. 2
1.3.4 Epidemiological triad ............................................................................................................. 3
1.3.5 Chain of infection ................................................................................................................... 3
1.4 Classification of Communicable Diseases .................................................................................... 7
1.5 Why Are Schools/Centre More Vulnerable to outbreaks ofcommunicable Diseases ................... 8
1.6 Principles of Control of Communicable Diseases ......................................................................... 8
1.7 Statutory Notifiable Communicable Diseases ............................................................................... 8
1.8 Definitions Related To Communicable Diseases .......................................................................... 9
1.9 Summary ..................................................................................................................................... 10
LECTURE TWO ................................................................................................................................... 11
DISEASE CAUSING ORGANISMS AND TROPICAL DISEASES .................................................. 11
2.1 Introduction ................................................................................................................................. 11
2.2 Objectives .................................................................................................................................... 11
2.3 Disease Causing Organisms ........................................................................................................ 11
iv
2.4 TROPICAL DISEASES .............................................................................................................. 12
2.4.1 Diseases Most Prevalent in the Tropics................................................................................ 12
2.4.2 Additional Health Problems ................................................................................................. 13
2.4.3 Factors Which Aggravate Tropical Diseases........................................................................ 13
2.5 Summary ..................................................................................................................................... 14
TROPICAL DISEASES: MALARIA, YELLOW FEVER AND DENGUE FEVER .......................... 15
3.1 Introduction ................................................................................................................................. 15
3.2 Objectives .................................................................................................................................... 15
3.3 MALARIA .................................................................................................................................. 15
3.3.1 What malaria is ..................................................................................................................... 15
3.3.2 How malaria is transmitted ................................................................................................... 16
3.3.3 Malaria distribution .............................................................................................................. 16
3.3.4 Signs and symptoms of malaria ............................................................................................ 16
3.3.5 Incubation period for malaria ............................................................................................... 17
3.3.6 Malaria diagnosis ................................................................................................................. 17
3.3.7 Malaria treatment.................................................................................................................. 17
3.3.8 Malaria and pregnancy ......................................................................................................... 18
3.3.9 Malaria and children ............................................................................................................. 19
3.3.10 How do you keep from getting malaria .............................................................................. 19
3.3.11 Precautions to take to avoid malaria ................................................................................... 19
3.4 YELLOW FEVER ...................................................................................................................... 19
3.4.1 Infectious agent that causes yellow fever ............................................................................. 20
3.4.2 Transmission ........................................................................................................................ 20
3.4.3 Incubation period .................................................................................................................. 20
3.4.4 Signs and symptoms of yellow fever.................................................................................... 20
3.4.5 Possible Complications ........................................................................................................ 21
3.4.6 Diagnosis of yellow fever ..................................................................................................... 21
3.4.7 Who is at risk for yellow fever ............................................................................................. 21
v
3.4.8 Distribution of yellow fever ................................................................................................. 21
3.4.9 Yellow fever as an emerging or re-emerging infectious disease .......................................... 21
3.4.10 Treatment............................................................................................................................ 22
3.4.11 Prevention ........................................................................................................................... 22
a) ..................................................................................................................................... Vaccination
....................................................................................................................................................... 22
b)............................................................................................................................. Mosquito control
....................................................................................................................................................... 22
3.4.12 Conclusion .......................................................................................................................... 23
3.5 DENGUE FEVER ....................................................................................................................... 23
3.5.1 What dengue fever is ............................................................................................................ 23
3.5.2 Distribution ........................................................................................................................... 24
3.5.3 Transmission ........................................................................................................................ 24
3.5.4 Incubation period .................................................................................................................. 24
3.5.5 Symptoms and signs ............................................................................................................. 24
3.5.6 Treatment for dengue fever .................................................................................................. 25
3.5.7 Prognosis for typical dengue fever ....................................................................................... 25
3.5.8 Dengue hemorrhagic fever. .................................................................................................. 25
3.5.9 Prevention of dengue fever ................................................................................................... 26
3.5.10 Conclusion .......................................................................................................................... 26
3.6 Summary ..................................................................................................................................... 27
3.7 Self-Test Questions ..................................................................................................................... 28
LECTURE FOUR ................................................................................................................................. 29
TROPICAL DISEASES: TUBERCULOSIS (TB) AND CHOLERA .................................................. 29
4.1 Introduction ................................................................................................................................. 29
4.2 Objectives .................................................................................................................................... 29
4.3 TUBERCULOSIS (TB) .............................................................................................................. 29
4.3.1 What tuberculosis is ............................................................................................................. 29
4.3.2 Transmission of TB .............................................................................................................. 30
vi
4.3.3 What happens to the body when a person gets TB ............................................................... 30
4.3.4 Distribution and vulnerability for TB ................................................................................... 31
4.3.5 Signs and symptoms of tuberculosis .................................................................................... 31
4.3.6 Diagnosis tuberculosis .......................................................................................................... 31
4.3.7 Tuberculosis vaccine ............................................................................................................ 32
4.3.8 Treatment of tuberculosis ..................................................................................................... 32
4.3.9 Drug-resistant TB ................................................................................................................. 33
4.3.10 The future for TB................................................................................................................ 34
4.3.11 Conclusion .......................................................................................................................... 34
4.4 CHOLERA .................................................................................................................................. 35
4.4.1 Meaning of cholera. .............................................................................................................. 35
4.4.2 Signs and symptoms of cholera ............................................................................................ 35
4.4.3 Transmission of cholera ....................................................................................................... 35
4.4.4 Prevention of cholera............................................................................................................ 36
4.4.5 Cholera Vaccine ................................................................................................................... 36
4.4.6 Treatment of cholera............................................................................................................. 36
4.6 Summary ..................................................................................................................................... 36
4.7
Self-Test Questions ............................................................................................................... 37
LECTURE FIVE ................................................................................................................................... 38
TROPICAL DISEASES: TRYPANOSOMIASIS AND LEISHMANIASIS ....................................... 38
5.1 Introduction ................................................................................................................................. 38
5.2 Objectives .................................................................................................................................... 38
5.4 TRYPANOSOMIASIS (SLEEPING SICKNESS) ..................................................................... 38
5.4.1 Meaning of Trypanosomiasis .............................................................................................. 38
5.4.2 Causes of Trypanosomiasis .................................................................................................. 38
5.4.3 Distribution of Trypanosomiasis .......................................................................................... 39
5.4.4 Is Trypanosomiasis Contagious ............................................................................................ 39
5.4. 5 Signs and Symptoms of the Disease .................................................................................... 39
vii
5.4.6 Chagas disease ...................................................................................................................... 40
5.4.7 Diagnosis Trypanosomiasis .................................................................................................. 40
5.4.8 Treatment of Trypanosomiasis. ............................................................................................ 40
5.4.9 Reasons why the disease is common in tropics .................................................................... 40
5.4.10 Consequences of Trypanosomiasis..................................................................................... 40
5.4.11 Prevention of Trypanosomiasis .......................................................................................... 41
5.5 LEISHMANIASIS ...................................................................................................................... 41
5.5.1 Meaning of leishmaniasis ..................................................................................................... 41
5.5.2 Signs and symptoms of cutaneous leishmaniasis ................................................................. 41
5.5.3 Signs and symptoms of visceral leishmaniasis ..................................................................... 41
5.5.4 Distribution of leishmaniasis. ............................................................................................... 41
5.5.5 Transmission of leishmaniasis .............................................................................................. 42
5.5.6 Who is at risk for leishmaniasis............................................................................................ 42
5.5.7 Recovery from infection ....................................................................................................... 42
5.5.8 How serious leishmaniasis can be if not treated ................................................................... 43
5.5.9 Diagnosis of leishmaniasis ................................................................................................... 43
5.5.10 Treatment leishmaniasis ..................................................................................................... 43
5.5.11 Prevention of leishmaniasis ................................................................................................ 43
5.5.11 Reinfection ......................................................................................................................... 44
5.5.11Conclusion ........................................................................................................................... 44
5.5 Summary ..................................................................................................................................... 44
5.5 Self-Test Questions ..................................................................................................................... 45
LECTURE SIX...................................................................................................................................... 46
TROPICAL DISEASES: AMOEBIC, BACILLARY DYSENTERY AND TETANUS. .................... 46
6.1 Introduction ................................................................................................................................. 46
6.2 Objectives .................................................................................................................................... 46
6.3 AMOEBIC DYSENTERY .......................................................................................................... 46
6.3.1 What amoebic dysentery is ................................................................................................... 46
viii
6.3.2 Causes................................................................................................................................... 46
6.3.3 Risk Factors .......................................................................................................................... 46
6.3.5 Symptoms of Amebic dysentery .......................................................................................... 47
6.3.6 Diagnosis .............................................................................................................................. 47
6.3.7 Treatment.............................................................................................................................. 47
6.3.7.1 Medications ....................................................................................................................... 47
6.3.7.2 Prevention .......................................................................................................................... 47
6.4.1 What bacillary dysentery is .................................................................................................. 48
6.4.2 Transmissiom ....................................................................................................................... 48
6.4.3 Treatment.............................................................................................................................. 48
6.5 TETANUS. .................................................................................................................................. 48
6.5.1 What Tetanus is .................................................................................................................... 48
6.5.2 Tetanus Causes ..................................................................................................................... 49
6.5.3 Tetanus Symptoms ............................................................................................................... 50
6.5.4 Diagnosis .............................................................................................................................. 50
6.5.5 Tetanus Treatment ................................................................................................................ 51
6.5.5.1 Self-Care at Home ............................................................................................................. 51
6.5.5.2 Medical Treatment............................................................................................................. 51
6.5.6 Prevention ............................................................................................................................. 51
6.5.7 Prognosis .............................................................................................................................. 52
6.5.8 Vaccine (Shot) Complications (Side Effects) ....................................................................... 52
6.6 Summary ..................................................................................................................................... 53
LECTURE SEVEN ............................................................................................................................... 55
TROPICAL DISEASES: LEPROSY AND YAW ............................................................................... 55
7.1 Introduction ................................................................................................................................. 55
7.2 Objectives .................................................................................................................................... 55
7.3 LEPROSY (HANSEN'S DISEASE) ........................................................................................... 55
7.3.1 What leprosy is ..................................................................................................................... 55
ix
7.3.2 Causes of leprosy.................................................................................................................. 56
7.3.3 Symptoms and signs of leprosy ............................................................................................ 56
7.3.4 Different forms (classifications) of leprosy .......................................................................... 56
7.3.5 Transmmission of leprosy .................................................................................................... 57
7.3.6 Leprosy diagnosis ................................................................................................................. 58
7.3.7 Treatment leprosy ................................................................................................................. 58
7.3.8 Prevention leprosy ................................................................................................................ 59
7.3.9 Leprosy in Kenya ................................................................................................................. 59
7.3.10 Conclusion .......................................................................................................................... 60
7.4 YAWS ......................................................................................................................................... 60
7.4.1 What yaws is ........................................................................................................................ 60
7.4.2 Causes of yaws ..................................................................................................................... 61
7.4.3 Transmission ........................................................................................................................ 61
7.4.4 Developmental stages in the course of yaws ........................................................................ 61
7.4.5 Diagnosis of yaws ................................................................................................................ 61
7.4.6 Treatment of yaws ................................................................................................................ 62
7.4.7 Yaws a serious public health problem .................................................................................. 62
7.4.8 Conclusion ............................................................................................................................ 63
7.5 Summary ..................................................................................................................................... 63
LECTURE EIGHT ................................................................................................................................ 65
HELMINTHIASIS ................................................................................................................................ 65
8.1 Introduction ................................................................................................................................. 65
8.2 Objectives .................................................................................................................................... 65
8.3 HELMINTHIASIS ...................................................................................................................... 65
8.4 Types of Infection ....................................................................................................................... 65
8.5 SCHISTOSOMIASIS .................................................................................................................. 66
8.5.1 What is schistosomiasis ........................................................................................................ 66
8.5.2 Infectious agent that causes schistosomiasis ........................................................................ 66
x
8.5.3 Distribution of schistosomiasis............................................................................................. 67
8.5.4 Transmission of schistosomiasis .......................................................................................... 68
8.5.5 Signs and symptoms of schistosomiasis ............................................................................... 68
8.5.6 Diagnosis of schistosomiasis ................................................................................................ 68
8.5.7 Who is at risk for schistosomiasis ........................................................................................ 68
8.5.8 Treatment of schistosomiasis ............................................................................................... 69
8.5.9 Complications resulting from schistosomiasis ..................................................................... 70
8.5.10 Schistosomiasis as an emerging infectious disease ............................................................ 70
8.5.11 Prevention and Control of Schistosomiasis ........................................................................ 70
8.5.12 Conclusion .......................................................................................................................... 71
8.6 ONCHOCERCIASIS (RIVER BLINDNESS) ............................................................................ 72
8.6.1 Infectious Agent ................................................................................................................... 72
8.6.2 Mode of Transmission .......................................................................................................... 72
8.6.3 Distribution ........................................................................................................................... 72
8.6.4 Risk for Travelers ................................................................................................................. 72
8.6.5 Signs and Symptoms ............................................................................................................ 72
8.6.6 Diagnosis .............................................................................................................................. 72
8.6.7 Treatment.............................................................................................................................. 73
8.6.8 Preventive Measures............................................................................................................. 73
8.7.1 Infectious agent that causes lymphatic filariasis .................................................................. 73
8.7.2 Distribution of lymphatic filariasis ....................................................................................... 73
8.7.3 How lymphatic filariasis is spread ....................................................................................... 73
8.7.4 Signs and symptoms of lymphatic filariasis ......................................................................... 74
8.7.5 Incubation period .................................................................................................................. 74
8.7.6 Diagnosis lymphatic filariasis .............................................................................................. 74
8.7.7 People at risk for lymphatic filariasis ................................................................................... 74
8.7.8 Complications resulting from lymphatic filariasis ............................................................... 74
8.7.9 Treatment for lymphatic filariasis ........................................................................................ 74
xi
8.7.10 How common lymphatic filariasis is. ................................................................................. 74
8.7.12 Lymphatic filariasis as an emerging infectious disease...................................................... 75
8.7.13 Prevention of lymphatic filariasis ....................................................................................... 75
8.7.14 Conclusion .......................................................................................................................... 75
8.8 ASCARIASIS .............................................................................................................................. 75
8.8.1 what Ascariasis is ................................................................................................................. 75
8.8.2 Transmission ........................................................................................................................ 75
8.8.3 Distribution ........................................................................................................................... 76
8.8.4 Life cycle .............................................................................................................................. 76
8.8.5 Transmission ........................................................................................................................ 77
8.8.6 Diagnosis .............................................................................................................................. 77
8.8.7 Symptoms ............................................................................................................................. 78
8.8.8 Treatment.............................................................................................................................. 78
8.8.9 Prevention ............................................................................................................................. 79
8.8.10 Trivia .................................................................................................................................. 79
8.9 TRICHURIASIS ......................................................................................................................... 80
8.9.1 Meaning Trichuriasis. ........................................................................................................... 80
8.9.2 Causes, incidence, and risk factors ....................................................................................... 80
8.9.3 Symptoms ............................................................................................................................. 80
8.9.4 Signs and tests ...................................................................................................................... 80
8.9.5 Treatment.............................................................................................................................. 80
8.9.6 Prognosis .............................................................................................................................. 81
8.9.7 Complications ....................................................................................................................... 81
8.9.8 Prevention ............................................................................................................................. 81
8.10 HOOKWORM........................................................................................................................... 81
8.10.1 What hookworm is ............................................................................................................. 81
8.10.2 Life cycle ............................................................................................................................ 82
8.10.3 Incubation Period................................................................................................................ 83
xii
8.10.4 Prevention ........................................................................................................................... 83
8.10.5 Symptoms ........................................................................................................................... 83
8.10.6 Diagnosis ............................................................................................................................ 84
8.10.7 Treatment............................................................................................................................ 84
8.10.8 Trivia .................................................................................................................................. 85
8.12 Summary ................................................................................................................................... 86
8.12 Self-Test Questions ................................................................................................................... 87
LECTURE NINE .................................................................................................................................. 88
TROPICAL DISEASES: DIARRHOEA AND TYPHOID FEVER. ................................................... 88
9.1 Introduction ................................................................................................................................. 88
9.2 Objectives .................................................................................................................................... 88
9.3 DIARRHOEA ............................................................................................................................. 88
9.3.1 What diarrhoea is.................................................................................................................. 88
9.3.2 Cause .................................................................................................................................... 89
9.3.3 Transmission ........................................................................................................................ 89
9.3.4 Distribution ........................................................................................................................... 89
9.3.5 Scope of the Problem............................................................................................................ 89
9.3.6 Signs and symptoms ............................................................................................................. 90
9.3.7 Diagnosis .............................................................................................................................. 90
9.3.8 Treatment and Interventions ................................................................................................. 91
9.3.9 Control and prevention ......................................................................................................... 92
9.4 TYPHOID FEVER .......................................................................................................................... 92
9.4.1 What typhoid fever is ........................................................................................................... 92
9.4.2 Causes and transmission ....................................................................................................... 92
9.4.3 Symptoms ............................................................................................................................. 92
9.4.4 Diagnosis .............................................................................................................................. 93
9.4.5 Treatment.............................................................................................................................. 93
9.4.6 Prognosis .............................................................................................................................. 93
xiii
9.4.7 Possible Complications ........................................................................................................ 93
9.4.8 Prevention ............................................................................................................................. 94
9.6 Summary ..................................................................................................................................... 94
9.7 Self-test questions........................................................................................................................ 95
LECTURE TEN .................................................................................................................................... 97
TROPICAL DISEASES: POLIOMYELITIS, MEASLES, DIPHTHERIA, ........................................ 97
WHOOPING COUGH .......................................................................................................................... 97
10.1 Introduction ............................................................................................................................... 97
10.2 Objectives .................................................................................................................................. 97
10.3 POLIOMYELITIS..................................................................................................................... 97
10.3.1 What poliomyelitis is .......................................................................................................... 97
10.3.2 Causes................................................................................................................................. 97
10.3.3 Incubation period ................................................................................................................ 97
10.3.4 Distribution ......................................................................................................................... 98
10.3.5 Symptoms ........................................................................................................................... 98
10.3.6 Diagnosis ............................................................................................................................ 99
10.3.7 Treatment............................................................................................................................ 99
10.3.8 Prognosis .......................................................................................................................... 100
10.3 9 Possible Complications .................................................................................................... 100
10.3.10 Prevention ....................................................................................................................... 100
10.4 MEASLES ............................................................................................................................... 101
10.4.1 What Measles is................................................................................................................ 101
10.4.2 Measles Causes................................................................................................................. 101
10.4.3 Measles Symptoms ........................................................................................................... 101
10.4.4 Diagnosis .......................................................................................................................... 102
10.4.5.1 Self-Care at Home ......................................................................................................... 103
10.4.5.2 Medical Treatment......................................................................................................... 103
10.4.6 Prevention ......................................................................................................................... 103
xiv
10.4.7 Prognosis .......................................................................................................................... 104
10.5 DIPHTHERIA ......................................................................................................................... 104
10.5.1 What diphtheria is............................................................................................................. 104
10.5.2 History of diphtheria......................................................................................................... 104
10.5.3 Causes of diphtheria ......................................................................................................... 105
10.5.4 Transmission .................................................................................................................... 105
10.5.5 Signs and symptoms of diphtheria ................................................................................... 105
10.5.6 Diagnosis .......................................................................................................................... 106
10.5.7 Treatment for diphtheria ................................................................................................... 106
10.9 Prevention of diphtheria .......................................................................................................... 107
10.5.10 Conclusion ...................................................................................................................... 107
10.6.1 What whooping cough is. ................................................................................................. 108
10.6.2 Prevention with a vaccine. ................................................................................................ 108
10.6.3 Symptoms, signs, and stages of whooping cough ............................................................ 109
10.6.4 Transmission .................................................................................................................... 110
10.6. 5 Adults and whooping cough ............................................................................................ 110
10.6.6 Diagnosis .......................................................................................................................... 110
10.6.7 Treatment for whooping cough. ....................................................................................... 110
10.6.8 Complications of whooping cough ................................................................................... 111
10.6.9 Conclusion ........................................................................................................................ 111
10.7 Summary ............................................................................................................................. 112
10.8 Self-Test Questions ................................................................................................................. 112
10.9 Further reading ........................................................................................................................ 113
LECTURE ELEVEN .......................................................................................................................... 114
SEXUALLY TRANSMITTED DISEASES (STDs) .......................................................................... 114
11.1 Introduction ............................................................................................................................. 114
12.2 Objectives ................................................................................................................................ 114
11.3 Overview of Sexually Transmitted Diseases (STDs) .............................................................. 114
xv
11.3.1 Sexually Transmitted Diseases (STDs) Causes ................................................................ 115
11.4 Sexually Transmitted Diseases (STDs) Symptoms ................................................................. 116
11.4.1 Symptoms of STDs caused by bacteria ............................................................................ 116
11.4.2 Symptoms of STDs caused by viruses ............................................................................. 118
11.4.3 Symptoms of STDs caused by protozoan ......................................................................... 119
11.4.4 Symptoms of STDs caused by fungi ................................................................................ 119
11.4.5 Symptoms of STDs caused by parasites ........................................................................... 120
11.4.6 Diagnosis .......................................................................................................................... 120
11.5 Sexually Transmitted Diseases (STDs) Treatment.................................................................. 120
11.5.1 Self-Care at Home ............................................................................................................ 120
11.6 Prevention................................................................................................................................ 121
11.7 Summary ................................................................................................................................. 121
11.8 Self-Test Questions ................................................................................................................. 122
11.9 Further reading ........................................................................................................................ 122
LECTURE TWELVE.......................................................................................................................... 123
EMERGING AND RE-EMERGING DISEASES .............................................................................. 123
12.1 Introduction ............................................................................................................................. 123
12.2 Objectives ................................................................................................................................ 123
12.3 Emerging and Re-Emerging Diseases ..................................................................................... 123
12.3.1 Emerging diseases ............................................................................................................ 123
12.3.2 Re-emerging, resurging diseases. ..................................................................................... 123
12.3.2 Factors which have contributed to emerging and re-emerging diseases. ......................... 123
12.4 HIV/AIDS ............................................................................................................................... 124
12.5 Malaria and Tuberculosis ........................................................................................................ 125
12.6 Influenza .................................................................................................................................. 126
12.7 SARS ....................................................................................................................................... 127
12.8 West Nile Virus ....................................................................................................................... 127
12.9 Marburg Virus ......................................................................................................................... 128
xvi
12.10 Rift valley fever. .................................................................................................................... 128
12.11 Bioterrorism........................................................................................................................... 129
12.12 Chikungunya. ........................................................................................................................ 129
12.12 Self-Test Questions ............................................................................................................... 129
LECTURE THIRTEEN ...................................................................................................................... 130
GENDER ASPECTS OF COMMUNICABLE DISEASES ............................................................... 130
13.1 Introduction ............................................................................................................................. 130
13.2 Objectives ................................................................................................................................ 130
13.3 Gender inequalities in tuberculosis (TB) ................................................................................. 130
13.4 Gender and HIV/AIDS ............................................................................................................ 130
13.5 Schistosomiasis, leishmaniasis, onchocerciasis, lymphatic filariasis, Chagas disease, African
trypanosomiasis and leprosy ........................................................................................................... 130
13.5.1 Schistosomiasis ................................................................................................................ 131
13.5.2 Leishmaniasis, onchocerciasis and lymphatic filariasis ................................................... 131
13.6 Summery ................................................................................................................................. 132
13.7 Self-Test Questions ................................................................................................................. 132
REFERENCES .................................................................................................................................... 133
xvii
xviii
LECTURE ONE
CONCEPTS, DEFINITIONS AND CLASSIFICATION
OF COMMUNICABLE DISEASES
1.1 Introduction
Welcome to the first lecture in our course. In this lecture we will cover concepts, definitions
and classifications of communicable diseases. The broader objective of this lecture is to
enable you to describe the concepts, definitions and classifications of communicable diseases.
1.2 Objectives
By end of this lecture you should be able to:
Describe the meaning of communicable diseases.
Describe the concepts communicable diseases.
Classification of communicable diseases.
1.3 Concepts of Communicable Diseases
1.3.1 Meaning of communicable diseases
Do you know what is meant by the term communicable
diseases? Write your answer on a piece of paper then
compare what you have written with the content below.
The term "communicable disease" refers to a disease that can be easily transmitted
(communicated) from a person or animal with the disease to a person or animal that
does not have the disease. Communicable diseases may be transmitted directly
(person-to-person contact) or transmitted indirectly (something carries the disease
organism from the diseased person to the healthy person). The term "infectious
disease" is sometimes used instead of "communicable disease." In this subcourse, both
terms will be used to mean the same thing
Communicable diseases are illnesses that spread from one person to another. They are
also called contagious or infectious diseases.
They are caused by infective agents (pathogens) that invade the body or release toxins
to cause damages to normal body cells and their functions. In severe cases, they may
lead to death.
1
1.3.2 Common Characteristics of Communicable Diseases
Think of any five characteristics of communicable diseases.
Then read the list below to see how many were correct.
I hope your answer included the following examples:
1.
2.
3.
4.
5.
They are very common.
Some of them cause death and disability.
Some of them cause epidemics.
Most of them are preventable when using fairly simple interventions.
Many of them affect infants and children.
1.3.3 Communicable disease cycle
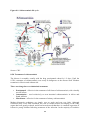
It is important to understand the cycle of communicable diseases (see Figure below).
This may help to identify the individuals that are likely to transmit the disease, as well
as those at greatest risk of becoming ill or dying within the population.
The Communicable Disease Cycle
2
Communicable diseases do not always develop in the same way in susceptible hosts. Some
diseases produce more non-clinical cases (e.g., polio, tuberculosis), while other diseases
produce more clinical cases (e.g.,measles). However, once exposed, even people without
clinical or biological signs of infection are capable of spreading the disease to other
susceptible hosts. Such people are known as carriers.
1.3.4 Epidemiological triad
The causative factors of disease are agent, host, and environment. These three factors are
referred as epidemiological triad. The mere presence of these factors is not sufficient to
initiate a disease. An interaction of all these three factors is necessary to initiate the disease
process. In pre pathogenesis phase, the disease agent is already present but it has not entered
man. The next phase is the Pathogenesis: this phase begins with the entry of disease agent into
man. There are certain interval of time before the onset of clinical signs and symptoms of the
disease. This period is called Incubation Period during this period the disease agent multiplies
and induces tissue and physiological changes. Incubation period is followed by early pre
pathogenesis. During this period, the signs and symptoms are not clear-cut. This is followed
by late pathogenesis when there are clear-cut signs and symptoms. The final outcome of the
disease may be recovery, disability or death. Each disease has its own natural history, but it is
not necessarily the same in all individuals. If the phase of natural history is known,
appropriate level of prevention can be applied.
1.3.5 Chain of infection
The chain of infection includes the three factors that lead to infection: the etiologic
agent, the method of transmission, and the host). These links should be characterized
before control and prevention measures are proposed. Environmental factors that may
influence disease occurrence must be evaluated.
As healthcare professionals, it is important to understand two things about infection:
-
the various ways infection can be transmitted
the ways the infection chain can be broken
Besides the infective agent, there are other crucial factors for the spread of
communicable diseases, namely the reservoir, portal of exit, portal of entry,
susceptible host, and mode of transmission. They are discussed below.
There are six links in the chain of infection. They are discussed below:
3
Figure: Chain of infection.
4
1.3.5.1 Infectious Agent
A microbial organism with the ability to cause disease. The greater the organism's
virulence (ability to grow and multiply), invasiveness (ability to enter tissue) and
pathogenicity (ability to cause disease), the greater the possibility that the organism
will cause an infection. Infectious agents are bacteria, virus, fungi, and parasites.
1.3.5.2 Reservoir
A place within which microorganisms can thrive and reproduce. For example,
microorganisms thrive in human beings, animals, and inanimate objects such as water,
table tops, and doorknobs.
1.3.5.3 Portal of Exit
A place of exit providing a way for a microorganism to leave the reservoir. For
example, the microorganism may leave the reservoir through the nose or mouth when
someone sneezes or coughs. Microorganisms, carried away from the body by feces,
may also leave the reservoir of an infected bowel.
1.3.5.4 Portal of Entry
An opening allowing the microorganism to enter the host. Portals include body
orifices, mucus membranes, or breaks in the skin. Portals also result from tubes placed
in body cavities, such as urinary catheters, or from punctures produced by invasive
procedures such as intravenous fluid replacement.
1.3.5.5 Susceptible Host
A person who cannot resist a microorganism invading the body, multiplying, and
resulting in infection. The host is susceptible to the disease, lacking immunity or
physical resistance to overcome the invasion by the pathogenic microorganism.
Susceptible host is an individual who has low resistance to particular disease. This
may be due to various factors such as;
-
Lack of previous contact with the disease hence no immune cells
Immuno suppressive illnesses such as AIDS
Malnutrition
Drugs that a person may be consuming.
1.3.5.6 Mode of Transmission
Method of transfer by which the organism moves or is carried from one place to
another. The hands of the health care worker may carry bacteria from one person to
another.
5
Mode of
transmission
Contact
Droplet
transmission
Air-borne
transmission
Process
Transmission Through direct body
contact with the infected persons, e.g.
playing together with direct skin
contacts; or indirect through contact
with objects contaminated by
infective agents, e.g. sharing towels,
combs and clothes
Inhale or contact of droplets expelled2
from the sick during sneezing,
3
coughing, spitting and speaking, or 4
through subsequent touching of
5
mucous membranes of the mouth, 6
nose and the eyes, etc with hands 7
contaminated with infective agents
The infective agents float in the air 8
for some time and enter the body 9
through the respiratory tract
10
Food-borne /water- Through ingestion of contaminated 11
borne transmission food or water, or use of contaminated12
eating utensils
13
14
15
16
Vector-borne
Through vectors, usually insects. The
transmission
infective agents parasitise and breed
in the bodies of the insects.
Blood / body fluid
transmission
Congenital
infection
Through blood transfusion, tattooing,17
ear piercing or sexual intercourse 18
19
From the pregnant mother to the
20
foetus
Examples of communicable
diseases
- Hand, foot and mouth
disease
- Acute conjunctivitis
- Head lice
- Scabies
- Chickenpox
Influenza
Common cold
Acute bronchiolitis
Pneumonia
Severe acute respiratory
syndrome (SARS)
Chickenpox
Measles
Pulmonary tuberculosis
Viral gastroenteritis
Food poisoning
Cholera
Bacillary dysentery
Hepatitis A
Hepatitis E
Mosquito-borne
Dengue fever
Malaria
Japanese encephalitis
Hepatitis B
Acquired immunodeficiency
syndrome (AIDS)
Congenital rubella syndrome
Note that: Some communicable diseases have more than one mode of transmissions (e.g.
chickenpox).
Note that: Disease transmission process has three components i.e. source, transmission route
and susceptible host. Source is the origin of the disease causing organism. This could be
infected person, animal, place or object. Transmission route - the main routes of transmission
are;
Direct contact for example sexual contact
Vectors like mosquitoes
Faecal oral (ingesting contaminated food and water)
Airbone
6
Transplacental (mother to foetus)
Blood contact (transfusion, surgery, injection)
Contact with animals or their products that are infected.
1.4 Classification of Communicable Diseases
We shall now focus on the various classes of communicable diseases. There are various ways
of classifying communicable diseases. The classification given below is considered to be the
most appropriate for ease of understanding. The detailed description of each of the classes
will be discussed in the respective units of this course.
Discuss with a colleague the classes of communicable
diseases listed below. Explore any other method of communicable
disease classifications that you might have read elsewhere.
The classes include:
Contact diseases such as scabies, pediculosis, fungal skin infections, trachoma, acute
bacterial conjunctivitis.
Sexually transmitted diseases and HIV/AIDS
Vector borne diseases such as relapsing fever, bancroftian filariasis, onchocerciasis,
yellow fever, trypanosomiasis, plague, schistosomiasis, dracunculosis, leishmaniasis
and malaria.
Diseases caused by Faecal – oral contamination such as acute gastro-enteritis,
bacillary dysentery, campylobacter jejuni, giadiasis, amoebiasis, cholera, enteric
fevers, food poisoning, poliomyelitis, viral hepatitis.
Helmonthic diseases such as ascariasis, enterobiasis, trichuriasis, hookworm,
strongyloidiasis, taeniasis, hydatidosis.
Airborne diseases such as acute respiratory infections, meningitis (bacterial and
fungal) tuberculosis and leprosy.
Zoonotic diseases (diseases of contact with animals or animal products) such as
anthax, brucellosis, rabies, hydatidosis, tetanus.
7
1.5 Why Are Schools/Centre More Vulnerable to outbreaks ofcommunicable Diseases?
Schools/centres are gathering places where children learn and play. Some children may
be too young to take proper personal care. As such, communicable diseases can easily
spread through close person-to-person contact.
The source of infection can be children, staff and parents. Person-to-person contact
may lead to cross-infection, i.e. the transmission of infective agents from one person to
another.
For example, a member of staff who fails to wash hands after caring for a sick child
before making contact with another child, he/she may spread the infective agents from
that child to the next child he/she cares for.
1.6 Principles of Control of Communicable Diseases
As mentioned above, there are four factors crucial to the spread of communicable
diseases. They include the infective agent, the source of infection, the mode of
transmission and the host.
Hence, the control of the spread of communicable diseases should focus on controlling
all these four factors so as to break the chain of infection.
Factors of transmission
Infective agents
Source of infection
Control measures
Disinfection to kill the infective agents
21 - Early detection, isolation and treatment
of patients
22 - Removal of breeding sites
Mode of transmission
23
24
25
26
Host (susceptible population)
27 - Building up personal immunity by
immunisation and healthy lifestyles
- Maintenance of good environmental,
personal and food hygiene
- Adoption of infection control measures
appropriate to the different modes of
transmission
1.7 What Are Statutory Notifiable Communicable Diseases?
Some communicable diseases are highly infectious and cause severe sequelae to such
an extent that they threaten human lives and affect the economy.
If there are proper precautionary or control measures in place, the disaster posed by
these communicable diseases can be averted.
The evolution of outbreaks of communicable diseases and their management vary to a
certain extent with different countries or regions, where the types of communicable
diseases occur and the living environment are different.
8
To safeguard public health and safety, every country or region has legislation
stipulating certain communicable diseases as statutory notifiable diseases that warrant
special precautions, and policies are developed to prevent outbreaks and contain their
spread.
1.8 Definitions Related To Communicable Diseases
"Communicable disease” means an illness due to a specific infectious agent or its
toxic products that arises through transmission of that agent or its products from an
infected person, animal, arthropod, or inanimate reservoir to a susceptible host, either
directly or indirectly, through an intermediate plant or animal host, vector, or the
inanimate environment.
“Carrier” means a person who harbors a specific infectious agent without discernible
clinical disease and serves as a potential source of infection.
“Case” means a person who harbors a communicable disease, usually in the presence
of discernible clinical disease, symptoms, or signs and may serve as a potential source
of infection.
“Contact” means a person or animal that has been in association with an infected
person or animal, or a contaminated environment that is likely to provide an
opportunity to acquire the infection.
“Emerging infectious diseases” means disease that may arise suddenly and/or
unexpectedly, including disease caused by antibiotic-resistant organisms.
“Health care worker” means a person who provides services whether as an
individual health care provider, volunteer, or student at or employee of a health care
facility.
“High risk sexual conduct” means unprotected sex with an individual or a group of
individuals with multiple partners.
“Latent Tuberculosis Infection” (LTBI) means infection with the tubercle bacillus
(the causative agent of tuberculosis) as evidenced by a positive tuberculin skin test but
having no evidence of active tuberculosis disease (i.e., clinical, radiological, and/or
microbiological).
“Medical laboratory” means an entity that engages in the biological, microbiological,
serological, chemical, immunohematological, radioimmunological, hematological,
cytological, pathological, or other examination of materials derived from the human
body for the detection, diagnosis, prevention, or treatment of any disease, infection, or
impairment, or the assessment of human health.
“Outbreak” means cases of disease occurring in a community, region, or particular
population at a rate clearly in excess of that which is normally expected.
“Quarantine” means the restriction of the activities or confinement of well persons or
animals who have, or may have been exposed to a case of communicable disease
during its period of communicability to prevent disease transmission during the
incubation period, if infection should occur.
“Restriction of activities” means limitations placed on the activities of persons with
disease or infection to prevent transmission of communicable diseases to other
individuals.
“Serious and present danger to health” means one (1) or more of the following: (A)
repeated behavior by a carrier or case that has been demonstrated epidemiologically to
transmit, or evidences a careless disregard for the transmission of the disease to others,
(B) a substantial likelihood that a carrier or case will repeatedly transmit the disease to
9
others as is evidenced by that individual’s past behavior, or by statements of the
individual that are credible indicators of the individual’s intention, (C) affirmative
misrepresentation by a carrier of his or her carrier status prior to engaging in any
behavior that has been epidemiologically demonstrated to transmit the disease, or (D)
failure or refusal to carry out the carrier’s or case’s duty to warn
“Suspect case” means a person whose medical history, signs, and symptoms suggest
that this person may be incubating or may be actively infected with some
communicable disease.
1.9 Summary
Communicable diseases refer to diseases that can be transmitted and make people ill. They are
caused by infective agents (pathogens), that invade the body or release toxins to cause
damages to normal body cells and their functions. In severe cases, they may lead to death.
Besides the infective agent, there are three crucial factors for the spread of communicable
diseases, namely the source of infection, the mode of transmission and the host-the so-called
“chain of infection”. Disease transmission process has three components i.e. source,
transmission route and susceptible host. Source is the origin of the disease causing organism.
This could be infected person, animal, place or object.
Transmission route the main routes of transmission are; Direct contact for example sexual
contact, Vectors like mosquitoes, Faecal oral (ingesting contaminated food and water),
Airbone, Transplacental (mother to foetus), Blood contact (transfusion, surgery, injection),
Contact with animals or their products that are infected, Susceptible host is an individual who
has low resistance to particular disease. This may be due to various factors such as; Lack of
previous contact with the disease hence no immune cells, Immuno suppressive illnesses such
as AIDS, Malnutrition, Drugs that a person may be consuming.
They can be classified as include: Contact diseases, Sexually transmitted diseases and
HIV/AIDS, Vector borne diseases, Diseases caused by Faecal – oral contamination,
Helmonthic diseases, Airborne diseases and Zoonotic diseases (diseases of contact with
animals or animal products)
1.10 Self-Test Questions
1. Define communicable disease.
2. List some of the characteristics of the communicable diseases.
3. Classify with clear example the various communicable diseases.
4. Why is it important to understand the chain of infection?
10
LECTURE TWO
DISEASE CAUSING ORGANISMS AND TROPICAL DISEASES
2.1 Introduction
Welcome to the second lecture in our course. In this lecture we will cover disease causing
organisms and tropical diseases. The broader objective of this lecture is to enable you to
describe disease causing organisms and tropical diseases.
2.2 Objectives
By end of this lecture you should be able to
i)
ii)
iii)
iv)
Describe disease causing organisms.
Describe tropical disaeses.
Describe diseases prevalent in the subtropical and tropical areas.
Explain the factors agrevating tropical diseases.
2.3 Disease Causing Organisms
Communicable diseases are caused by pathogens. The germs are so small that they can only
be seen with a microscope, not with the naked eye. The germs and bugs are categorized
below.
Bacteria
Fungi
Parasites
Viruses
• E. coli [often the culprit for urinary tract infections in females
• Streptococcus [the famous ‘strep throat’ that is scratchy & raw]
• Staphylococcus [better know as ‘staph infections’ with wounds]
• Tuberculosis
• Thrush which is found in the mouth (internal)is often seen as a white thick
coating on the tongue
• Candidiasis which is found in the vaginal tract (internal) is what we know
as a yeast infection often seen as a curd-like white discharge
• Ringworm is found on the skin affecting more commonly the arms, legs or
chest area (external), but can be anywhere and seen as round, ring-like
patches of crusty areas on the skin
• Tapeworms (from uncooked meat; eating raw meat is still popular in
restaurants today; although many are publishing warnings on their menus
regarding consumption of undercooked meats)
• Lice ( “the nits” in body hairs; commonly in the scalp or pubic area)
• Giardia (from a contaminated water source such as a river or lake that can
give an individual unrelenting liquid diarrhea stools)
• Hepatitis or HIV (blood borne)
11
• Influenza (that winter flu) or RSV in infants (respiratory)
• Venereal warts (sexually transmitted)
• Chicken pox (contact)
Note: the following factors contribute so much to the pathogenic infectivity including
- Transmissibility
- Usual reservoir
- Host range
- Route of transmission
- Rate of replication/growth
- Virulence
- Predilection site in host
2.4 TROPICAL DISEASES
Tropical diseases are illnesses that either occurs uniquely in tropical and subtropical
regions (which is rare) or, more commonly, are either more widespread in the tropics
or more difficult to prevent or control.
The tropics are more problematic for certain diseases for two reasons:
1) Tropical climates are more conducive to certain diseases.
2) Areas of poverty and primitive sanitation conditions are more common in
the tropics.
2.4.1 Diseases Most Prevalent in the Tropics
The most important diseases in the tropical regions of Southeast Asia, Africa, and
South America are malaria, schistosomiasis, leprosy, filariasis, trypanosomiasis, and
leishmaniasis.
Although effective chemotherapy and insecticides have reduced or eliminated malaria
in most of the western hemisphere, these measures have been less successful in in
other areas like Asia. Both the infecting parasite and its mosquito carrier have become
resistant to current drugs.
200 million persons are estimated to have malaria in tropical areas. In sub-Saharan
Africa some 1 million children under five die of the disease each year.
Schistosomiasis has never been common in temperate climates, but it affects 125
million persons worldwide, of whom approximately 20 percent are at least partly
disabled by the disease. Praziquantel, a highly effective new drug, is now available for
treatment of schistosomiasis.
Leprosy has also always been more common in tropical than in moderate climates,
and about 11 million persons in the world have this illness. In endemic areas many
12
severe cases of leprosy are now resistant to the drug first used against it, and newer,
more expensive therapy must be employed.
Filariasis is a common tropical debilitating illness caused by infection with
roundworm larvae.
Trypanosomiasis, which results from infection with a protozoan parasite, has caused
10 million cases of human sickness in Africa alone. A related protozoan in South
America causes a less deadly form of trypanosomiasis called Chagas' disease.
Leishmaniasis is also a result of worm infection, and in its Asian and African forms
the disease can damage the internal organs.
2.4.2 Additional Health Problems
Although tuberculosis is largely under control in developed countries, it is still a
considerjable public health problem in much of the world and is responsible for about
half a million deaths annually, 75 percent of them in Asia.
Other diseases for which treatment is available but which are still common in
developing countries include cholera, yellow fever, yaws, and amoebic dysentery
Two forms of cancer, Burkitt's lymphoma and liver cancer are very common in Africa
and Asia, respectively, although rare in temperate zones. Burkitt's lymphoma is
thought to be due to a combination of massive infection with a virus early in life and
malaria in adulthood. Liver cancer may be caused by infection with the hepatitis B
virus.jun
As many as 25 million persons have become blind from preventable diseases in
tropical countries. These diseases include xerophthalmia, due to lack of vitamin A in
the diet; onchocerciasis, or river blindness, an infection of the skin by filarial larvae
that may also affect the conjunctiva of the eye; and trachoma, a chronic conjunctival
infection caused by the parasitic bacterium Chlamydia trachomatis, which is
transmitted by flies or through close personal contact.
Finally, a number of severe virus-caused fevers that were identified during the 1970s
are found predominantly in tropical regions. These diseases include Lassa, Ebola,
Marburg, Bunya, and Chikungunya fevers, some of which cause death by
hemorrhage (hemorrhagic fever). One member of this family, dengue virus (Dengue
Fever), was known for many years but has recently spread to the Caribbean and
Mexico. All these diseases are rare.
2.4.3 Factors Which Aggravate Tropical Diseases
The severity of diseases in tropical areas is due to widespread poverty and poor
sanitation as well as climatic influences. That is, because of low national incomes,
most developing countries cannot afford to buy vaccines to prevent poliomyelitis,
measles, and yellow fever. Only about 10 percent of the 80 million children in poor
countries have been immunized against diphtheria, whooping cough, and tetanus, and
such countries cannot afford to distribute drugs against tuberculosis or leprosy.
Poverty is a condition that also leads to malnutrition, which makes people more
susceptible to disease.
Poor sanitation is especially to blame for the spread of cholera, in which the infecting
agent is transmitted through contaminated sewage; and schistosomiasis, in which the
intermediate vector, a snail, lives in contaminated water.
13
Climate indirectly makes disease in tropical regions more severe by reducing
agricultural production, which increases the risk of malnutrition. In a more direct way,
hot weather and humid forests favor growth of the flies and mosquitoes that transmit
malaria, yellow fever, dengue fever, trachoma, trypanosomiasis, and onchocerciasis.
2.5 Summary
Communicable diseases are illnesses that spread from one person to another. They are also
called contagious or infectious diseases.
Communicable diseases are caused by pathogens. The germs are so small that they can only
be seen with a microscope, not with the naked eye. The germs and bugs are categorized as:
viruses (e.g., "colds," chicken pox, hepatitis A & B, HIV),
bacteria (e.g., "strep," tuberculosis), or
fungi (e.g., ringworm, thrush), and
Parasites (e.g., giardia, pinworms, scabies, head lice).
Illnesses caused by bacteria, fungi and parasites always need medical evaluation and
antibiotic treatment. Many illnesses caused by viruses are mild and go away on their own, but
some need medical evaluation and treatment.
2.6 Self-Test Questions
1. Think about the last communicable disease that "went around" your nearby
community. What was the impact on children, families and community?
How many got sick?
How much pain and suffering did they experience?
How many days of school or work were missed?
What were the costs for health care and medicine?
What might have been done to prevent or manage the illness more
effectively?
2. Describe diseases caused by each of the disease causing micro-organisms.
14
LECTURE THREE
TROPICAL DISEASES: MALARIA, YELLOW FEVER AND DENGUE FEVER
3.1 Introduction
Welcome to the third lecture in our course. In this lecture we will cover tropical diseases –
malaria, yellow fever and dengue fever. The broader objective of this lecture is to enable you
to describe tropical diseases – malaria, yellow fever and dengue fever.
3.2 Objectives
By end of this lecture you should be able to
i) Describe causes, signs &symptoms, prevention and control of malaria.
ii) Describe causes, signs &symptoms, prevention and control of yellow
fever.
iii) Describe causes, signs &symptoms, prevention and control of dengue
fever.
3.3 MALARIA
As you read through get the answers to the following questions:
What is malaria?
How is malaria transmitted?
Where is malaria a particular problem?
What are the signs and symptoms of malaria?
What is the incubation period for malaria?
How is malaria diagnosed?
How is malaria treated?
Is malaria a particular problem during pregnancy?
Is malaria a particular problem for children?
How do I keep from getting malaria?
What precautions should one take to avoid malaria?
3.3.1 What is malaria?
Malaria is an infectious disease caused by a parasite, Plasmodium, which infects red blood
cells. Malaria is characterized by cycles of chills, fever, pain and sweating. Historical records
suggest malaria has infected humans since the beginning of mankind.
The name "mal 'aria" (meaning "bad air" in Italian) was first used in English in 1740 by H.
Walpole when describing the disease. The term was shortened to "malaria" in the 20th
century. C. Laveran in 1880 was the first to identify the parasites in human blood.
15
In 1889, R. Ross discovered that mosquitoes transmitted malaria. Of the four species of
parasites that cause malaria, the most serious type is Plasmodium falciparum malaria. It can
be life-threatening. The other three species of malaria (P. vivax, P. malariae, and P. ovale) are
generally less serious and are not life-threatening.
3.3.2 How malaria is transmitted
The life cycle of the parasite is complicated and involves two hosts, humans and Anopheles
mosquitoes. The disease is transmitted to humans when an infected Anopheles mosquito bites
a person and injects the malaria parasites (sporozoites) into the blood.
Sporozoites travel through the bloodstream to the liver, mature, and eventually infect the
human red blood cells. While in red blood cells, the parasites again develop until a mosquito
takes a blood meal from an infected human and ingests human red blood cells containing the
parasites. Then the parasites reach the female Anopheles mosquito's stomach and eventually
invade the mosquito salivary glands.
When an Anopheles mosquito bites a human, these sporozoites complete and repeat the
complex Plasmodium life cycle. P. ovale and P. vivax can further complicate the cycle by
producing dormant stages (hypnozoites) that may not develop for weeks to years.
3.3.3 Malaria distribution
Malaria is a particular problem and a major one in areas of Asia, Africa, and Central and
South America. Unless precautions are taken, anyone living in or traveling to a country where
malaria is present can get the disease.
Malaria occurs in about 100 countries; approximately 40% of the world population is at risk
for contracting malaria.
3.3.4 Signs and symptoms of malaria?
The symptoms characteristics of malaria include:
•
•
•
•
Flu-like illness with fever,
Chills,
Muscle aches, and
Headache.
Some patients develop:
•
•
•
•
•
•
Nausea,
Vomiting,
Cough, and
Diarrhea.
Cycles of chills, fever, and sweating that repeat every one, two, or three days are
typical.
There can sometimes be vomiting, diarrhea, coughing, and yellowing (jaundice) of the
skin and whites of the eyes due to destruction of red blood cells and liver cells.
16
People with severe P. falciparum malaria can develop:
•
•
•
•
•
•
•
Bleeding problems,
Shock,
Liver or kidney failure,
Central nervous system problems,
Coma, and can die from the infection or its complications.
Cerebral malaria (coma, or altered mental status or seizures) can occur with severe P.
falciparum infection.
It is lethal if not treated quickly; even with treatment, about 15%-20% die.
3.3.5 Incubation period for malaria
The period between the mosquito bite and the onset of the malarial illness is usually one to
three weeks (seven to 21 days). This initial time period is highly variable as reports suggest
that the range of incubation periods may range from four days to one year.
The usual incubation period may be increased when a person has taken an inadequate course
of malaria prevention medications.
Certain types of malaria (P. vivax and P. ovale) parasites can also take much longer, as long
as eight to 10 months, to cause symptoms. These parasites remain dormant (inactive or
hibernating) in the liver cells during this time.
Unfortunately, some of these dormant parasites can remain even after a patient recovers from
malaria, so the patient can get sick again. This situation is termed relapsing malaria.
3.3.6 How is malaria diagnosed?
Clinical symptoms listed above, when associated with travel to areas that have identified
malarial risk, suggest malaria as a diagnosis. Malaria tests are not routinely ordered by most
physicians in developed countries so recognition of travel history is essential.
The classic and most used test is the blood smear on a microscope slide that is stained
(Giemsa stain) to show the parasites inside red blood cells. Although this test is easily done,
correct results are dependent on the technical skill of the lab technician who prepares and
examines the slides with a microscope.
Other tests based on immunologic principles exist, including RDT's (rapid diagnostic tests)
approved for use and the polymerase chain reaction (PCR) tests. These are not yet widely
available and are more expensive than the traditional Giemsa blood smear. Some investigators
suggest such immunologic based tests be confirmed with a Giemsa blood smear.
3.3.7 Malaria treatment
Three main factors determine treatments:
i.
ii.
The infecting species of Plasmodium parasite,
The clinical situation of the patient (for example, adult, child, or pregnant female with
either mild or severe malaria), and
17
iii.
The drug susceptibility of the infecting parasites.
Drug susceptibility is determined by the geographic area where the infection was acquired.
Different areas of the world have malaria types that are resistant to certain medications. The
correct drugs for each type of malaria must be prescribed by a doctor who is familiar with
malaria treatment protocols. Since people infected with P. falciparum malaria can die (often
because of delayed treatment), immediate treatment for P. falciparum malaria is necessary.
Mild malaria can be treated with oral medication; severe malaria (one or more symptoms of
either impaired consciousness/coma, severe anemia, renal failure, pulmonary edema, acute
respiratory distress syndrome, shock, disseminated intravascular coagulation, spontaneous
bleeding, acidosis, hemoglobinuria [hemoglobin in the urine], jaundice, repeated generalized
convulsions, and/or parasitemia [parasites in the blood] of > 5%) requires intravenous (IV)
drug treatment and fluids.
Drug treatment of malaria is not always easy. Chloroquine phosphate is the drug of choice for
all malarial parasites except for chloroquine-resistant Plasmodium strains. Although almost all
strains of P. malariae are susceptible to chloroquine, P. falciparum, P. vivax and even some P.
ovale strains have been reported as resistant to chloroquine. Unfortunately, resistance is
usually noted by drug-treatment failure in the individual patient. There are, however, multiple
drug-treatment protocols for treatment of drug resistant Plasmodium strains (for example,
quinine sulfate plus doxycycline [Vibramycin, Oracea, Adoxa, Atridox] or tetracycline
[Achromycin], or clindamycin [Cleocin], or atovaquone-proguanil [Malarone]). There are
specialized labs that can test the patient's parasites for resistance, but this is not done
frequently.
Consequently, treatment is usually based on the majority of Plasmodium species diagnosed
and its general drug-resistance pattern for the country or world region where the patient
became infested. For example, P. falciparum acquired in the Middle East countries is usually
susceptible to chloroquine, but if acquired in sub-Sahara African countries, is usually resistant
to chloroquine.
3.3.8 Malaria and pregnancy
Malaria may pose a serious threat to a pregnant woman and her pregnancy. Malaria infection
in pregnant women may be more severe than in women who are not pregnant.
Malaria may also increase the risk of problems with the pregnancy, including prematurity,
abortion, and stillbirth. Statistics indicate that in sub-Saharan Africa, between 75,000-200,000
infants die from malaria per year; worldwide estimates indicate over 1 million children die
from malaria each year.
Therefore, all pregnant women who are living in or traveling to a malaria-risk area should
consult a doctor and take prescription drugs (for example, sulfadoxine-pyrimethamine) to
avoid contracting malaria.
Treatment of malaria in the pregnant female is similar to the usual treatment described above;
however, drugs such as primaquine (Primaquine), tetracycline (Achromycin, Sumycin),
doxycycline, and halofantrine (Halfan) are not recommended as they may harm the fetus.
18
3.3.9 Malaria and children
All children, including young infants, living in or traveling to malaria-risk areas should take
antimalarial drugs (for example, chloroquine and mefloquine [Lariam]). Although the
recommendations for most antimalarial drugs are the same as for adults, it is crucial to use the
correct dosage for the child.
The dosage of drug depends on the age and weight of the child. Since an overdose of an
antimalarial drug can be fatal, all antimalarial (and all other) drugs should be stored in
childproof containers well out of the child's reach.
3.3.10 How do you keep from getting malaria?
If you are traveling to an area known to have malaria, find out which medications you need to
take, and take them as prescribed. It is recommended that individuals begin taking
antimalarial drugs about one to two weeks before traveling to a malaria infested area and for
four weeks after leaving the area.
Your doctor, travel clinic, or the health department can advise you as to what medicines to
take to keep from getting malaria.
Currently, there is no vaccine available for malaria, but researchers are trying to develop one.
3.3.11 Precautions to take to avoid malaria
The following precautions can be taken in malaria infested areas:
Avoid exposure to mosquitoes during the early morning and early evening hours
between the hours of dusk and dawn (the hours of greatest mosquito activity).
Wear appropriate clothing (long-sleeved shirts and long pants, for examples)
especially when you are outdoors.
Apply insect repellent to the exposed skin. Insect repellent should contains up to 50%
DEET (N,N-diethyl-m-toluamide), which is the most effective mosquito repellent for
adults and children over 2 months of age.
Spray mosquito repellents on clothing to prevent mosquitoes from biting through thin
clothing.
Use a permethrin-coated (or similar repellant) mosquito net over your all beds.
Have screens over cover windows and doors.
Spray permethrin or a similar insecticide in the bedroom before going to bed.
Use of long lasting treated mosquito net
3.4 YELLOW FEVER
Yellow fever is an acute viral haemorrhagic disease transmitted by infected
mosquitoes. The "yellow" in the name refers to the jaundice that affects some patients.
Yellow fever infection can cause severe illness and death. Up to 50% of severely
affected persons without treatment will die from yellow fever.
There are an estimated 200 000 cases of yellow fever, causing 30 000 deaths,
worldwide each year.
19
The virus is endemic in tropical areas of Africa and Latin America, with a combined
population of over 900 million people.
The number of yellow fever cases has increased over the past two decades due to
declining population immunity to infection, deforestation, urbanization, population
movements and climate change.
There is no cure for yellow fever. Treatment is symptomatic, aimed at reducing the
symptoms for the comfort of the patient.
Vaccination is the single most important preventive measure against yellow fever. The
vaccine is safe, affordable and highly effective, and appears to provide protection for
30–35 years or more. The vaccine provides effective immunity within one week for
95% of persons vaccinated.
3.4.1 Infectious agent that causes yellow fever
Yellow fever is caused by the yellow fever virus.
3.4.2 Transmission
The yellow fever virus is an arbovirus of the flavivirus genus, and the mosquito is the primary
vector. It carries the virus from one host to another, primarily between monkeys, from
monkeys to humans, and from person to person.
Several different species of the Aedes and Haemogogus mosquitoes transmit the virus. The
mosquitoes either breed around houses (domestic), in the jungle (wild) or in both habitats
(semi-domestic). There are three types of transmission cycles.
Sylvatic (or jungle) yellow fever: In tropical rainforests, yellow fever occurs in
monkeys that are infected by wild mosquitoes. The infected monkeys then pass the
virus to other mosquitoes that feed on them. The infected mosquitoes bite humans
entering the forest, resulting in occasional cases of yellow fever. The majority of
infections occur in young men working in the forest (e.g. for logging).
Intermediate yellow fever: In humid or semi-humid parts of Africa, small-scale
epidemics occur. Semi-domestic mosquitoes (that breed in the wild and around
households) infect both monkeys and humans. Increased contact between people and
infected mosquitoes leads to transmission. Many separate villages in an area can suffer
cases simultaneously. This is the most common type of outbreak in Africa. An
outbreak can become a more severe epidemic if the infection is carried into an area
populated with both domestic mosquitoes and unvaccinated people.
Urban yellow fever: Large epidemics occur when infected people introduce the virus
into densely populated areas with a high number of non-immune people and Aedes
mosquitoes. Infected mosquitoes transmit the virus from person to person.
3.4.3 Incubation period
Symptoms start 3 to 6 days after being bitten by an infected mosquito.
3.4.4 Signs and symptoms of yellow fever
ArrhythmiasArrhythmias, heart dysfunction
20
Bleeding (may progress to hemorrhage)
Coma
Decreased urinationDecreased urination
Delirium
Fever
Headache
JaundiceJaundice
Muscle aches (myalgia)
Red eyesRed eyes, face, tongue
Seizures
Vomiting
Vomiting bloodVomiting blood
3.4.5 Possible Complications
Coma
Death
Disseminated intravascular coagulation (DIC)Disseminated intravascular coagulation
(DIC)
Kidney failureKidney failure
Liver failure
ParotitisParotitis
Secondary bacterial infectionsSecondary bacterial infections
ShockShock
3.4.6 Diagnosis of yellow fever
Yellow fever is diagnosed by a blood test.
3.4.7 Who is at risk for yellow fever?
People are at risk if they travel to an area where there is yellow fever in humans or monkeys
and there are mosquitoes to spread the virus.
3.4.8 Distribution of yellow fever
Yellow fever is common in West and Central Africa and in parts of South America. Periodic
epidemics in Africa lead to hundreds of thousands of cases. Yellow fever is a very rare cause
of illness.
3.4.9 Yellow fever as an emerging or re-emerging infectious disease
There has been a dramatic re-emergence of yellow fever in Africa and South America since
the 1980s.
21
3.4.10 Treatment
There is no specific treatment for yellow fever, only supportive care to treat dehydration and
fever. Associated bacterial infections can be treated with antibiotics. Supportive care may
improve outcomes for seriously ill patients, but it is rarely available in poorer areas.
3.4.11 Prevention
a) Vaccination
Vaccination is the single most important measure for preventing yellow fever. In high risk
areas where vaccination coverage is low, prompt recognition and control of outbreaks through
immunization is critical to prevent epidemics. To prevent outbreaks throughout affected
regions, vaccination coverage must reach at least 60% to 80% of a population at risk. Few
countries in Africa currently have this level of coverage.
Preventive vaccination can be offered through routine infant immunization and one-time mass
campaigns to increase vaccination coverage in countries at risk, as well as for travelers to
yellow fever endemic area. WHO strongly recommends routine yellow fever vaccination for
children in areas at risk for the disease.
The yellow fever vaccine is safe and affordable, providing effective immunity against yellow
fever within one week for 95% of those vaccinated. A single dose provides protection for 30–
35 years or more, and probably for life. Serious side effects are extremely rare. Serious
adverse events have been reported rarely following immunization in a few endemic areas and
among vaccinated travelers (e.g. in Brazil, Australia, the United States, Peru and Togo).
Scientists are investigating the causes.
The risk of death from yellow fever is far greater than the risks related to the vaccine. People
who should not be vaccinated include:
children aged less than 9 months for routine immunization (or less than 6 months
during an epidemic);
pregnant women – except during a yellow fever outbreak when the risk of infection is
high;
people with severe allergies to egg protein; and
People with severe immunodeficiency due to symptomatic HIV/AIDS or other causes,
or in the presence of a thymus disorder.
Travelers, particularly those arriving to Asia from Africa or Latin America must have a
certificate of yellow fever vaccination. If there are medical grounds for not getting vaccinated,
International Health Regulations state that this must be certified by the appropriate authorities.
b) Mosquito control
Mosquito control is vital until vaccination takes effect. The risk of yellow fever transmission
in urban areas can be reduced by eliminating potential mosquito breeding sites and applying
insecticides to water where they develop in their earliest stages. Application of spray
insecticides to kill adult mosquitoes during urban epidemics, combined with emergency
22
vaccination campaigns, can reduce or halt yellow fever transmission, "buying time" for
vaccinated populations to build immunity.
Historically, mosquito control campaigns successfully eliminated Aedes aegypti, the urban
yellow fever vector, from most mainland countries of central and South America. However,
this mosquito species has re-colonized urban areas in the region and poses a renewed risk of
urban yellow fever.
Mosquito control programmes targeting wild mosquitoes in forested areas are not practical for
preventing jungle (or sylvatic) yellow fever transmission.
3.4.12 Conclusion
Yellow fever is a tropical disease that is spread to humans by infected mosquitoes.
Many yellow fever infections are mild, but the disease can cause severe, lifethreatening illness.
Yellow fever is found only in Africa and South America.
Yellow fever is preventable by immunization. Travelers to countries with yellow fever
should get the yellow fever vaccine.
3.5 DENGUE FEVER
As you read through try to get answers to the following questions
What is dengue fever?
What areas are at high risk for contracting dengue fever?
How is dengue fever contracted?
What are dengue fever symptoms and signs?
What is the treatment for dengue fever?
What is the prognosis for typical dengue fever?
What is dengue hemorrhagic fever?
How can dengue fever be prevented?
3.5.1 What dengue fever is
Dengue fever is a disease caused by a family of viruses that are transmitted by mosquitoes. It
is an acute illness of sudden onset that usually follows a benign course with symptoms such as
headache, fever, exhaustion, severe muscle and joint pain, swollen glands (lymphadenopathy),
and rash. The presence (the "dengue triad") of fever, rash, and headache (and other pains) is
particularly characteristic of dengue. Other signs of dengue fever include bleeding gums,
severe pain behind the eyes, and red palms and soles.
Dengue strikes people with low levels of immunity. Because it is caused by one of four
serotypes of virus, it is possible to get dengue fever multiple times. However, an attack of
23
dengue produces immunity for a lifetime to that particular serotype to which the patient was
exposed.
Dengue goes by other names, including "breakbone" or "dandy fever." Victims of dengue
often have contortions due to the intense joint and muscle pain, hence the name breakbone
fever. Slaves in the West Indies who contracted dengue were said to have dandy fever
because of their postures and gait.
Dengue hemorrhagic fever is a more severe form of the viral illness. Manifestations include
headache, fever, rash, and evidence of hemorrhage in the body. Petechiae (small red or purple
blisters under the skin), bleeding in the nose or gums, black stools, or easy bruising are all
possible signs of hemorrhage. This form of dengue fever can be life-threatening and can
progress to the most severe form of the illness, dengue shock syndrome.
3.5.2 Distribution
Dengue is prevalent throughout the tropics and subtropics. Outbreaks have occurred
recently in the Caribbean, including Puerto Rico, the U.S. Virgin Islands, Cuba, and
Central America.
Cases have also been imported via tourists returning from areas with widespread
dengue, including Tahiti, Singapore, the South Pacific, Southeast Asia, the West
Indies, India, and the Middle East (similar in distribution to the areas of the world that
harbor malaria and yellow fever).
Dengue fever is common, and statistics show it may be increasing in Southeast Asia.
Thailand, Vietnam, Singapore, and Malaysia have all reported an increase in cases.
According to the U.S. Centers for Disease Control and Prevention (CDC), there are an
estimated 100 million cases of dengue fever with several hundred thousand cases of
dengue hemorrhagic fever requiring hospitalization each year.
Nearly 40% of the world's population lives in an area endemic with dengue.
3.5.3 Transmission
The virus is contracted from the bite of a striped Aedes aegypti mosquito that has
previously bitten an infected person. The mosquito flourishes during rainy seasons but
can breed in water-filled flower pots, plastic bags, and cans year-round. One mosquito
bite can inflict the disease.
The virus is not contagious and cannot be spread directly from person to person. There
must be a person-to-mosquito-to-another-person pathway.
3.5.4 Incubation period
After being bitten by a mosquito carrying the virus, the incubation period ranges from
three to 15 (usually five to eight) days before the signs and symptoms of dengue
appear.
3.5.5 Symptoms and signs
Dengue starts with:
24
-
chills,
headache,
Pain upon moving the eyes, and
Low backache.
Painful aching in the legs and joints occurs during the first hours of illness.
The temperature rises quickly as high as 104 F (40 C), with relative low heart rate
(bradycardia) and low blood pressure (hypotension).
The eyes become reddened.
A flushing or pale pink rash comes over the face and then disappears.
The glands (lymph nodes) in the neck and groin are often swollen.
Fever and other signs of dengue last for two to four days, followed by a rapid drop
in body temperature (defervescence) with profuse sweating.
This precedes a period with normal temperature and a sense of well-being that
lasts about a day.
A second rapid rise in temperature follows.
A characteristic rash appears along with the fever and spreads from the extremities
to cover the entire body except the face.
The palms and soles may be bright red and swollen.
3.5.6 Treatment for dengue fever
Because dengue fever is caused by a virus, there is no specific medicine or antibiotic
to treat it.
For typical dengue, the treatment is purely concerned with relief of the symptoms
(symptomatic).
Rest and fluid intake for adequate hydration is important.
Aspirin and nonsteroidal anti-inflammatory drugs should only be taken under a
doctor's supervision because of the possibility of worsening hemorrhagic
complications.
Acetaminophen (Tylenol) and codeine may be given for severe headache and for the
joint and muscle pain (myalgia).
3.5.7 Prognosis for typical dengue fever
Typical dengue is fatal in less than 1% of cases.
The acute phase of the illness with fever and myalgias lasts about one to two weeks.
Convalescence is accompanied by a feeling of weakness (asthenia), and full recovery
often takes several weeks.
3.5.8 Dengue hemorrhagic fever.
Dengue hemorrhagic fever (DHF) is a specific syndrome that tends to affect children under 10
years of age. It causes abdominal pain, hemorrhage (bleeding), and circulatory collapse
(shock). DHF is also called Philippine, Thai, or Southeast Asian hemorrhagic fever and
dengue shock syndrome.
DHF starts abruptly with high continuous fever and headache. There are respiratory and
intestinal symptoms with sore throat, cough, nausea, vomiting, and abdominal pain. Shock
occurs two to six days after the start of symptoms with sudden collapse, cool, clammy
25
extremities (the trunk is often warm), weak pulse, and blueness around the mouth (circumoral
cyanosis).
In DHF, there is bleeding with easy bruising, blood spots in the skin (petechiae), spitting up
blood (hematemesis), blood in the stool (melena), bleeding gums, and nosebleeds (epistaxis).
Pneumonia is common, and inflammation of the heart (myocarditis) may be present.
Patients with DHF must be monitored closely for the first few days since shock may occur or
recur precipitously (dengue shock syndrome). Cyanotic (bluish) patients are given oxygen.
Vascular collapse (shock) requires immediate fluid replacement. Blood transfusions may be
needed to control bleeding.
The mortality (death) rate with DHF is significant. It ranges from 6%-30%. Most deaths occur
in children. Infants under a year of age are especially at risk of dying from DHF.
3.5.9 Prevention of dengue fever
The transmission of the virus to mosquitoes must be interrupted to prevent the illness. To this
end, patients are kept under mosquito netting until the second bout of fever is over and they
are no longer contagious.
The prevention of dengue requires control or eradication of the mosquitoes carrying the virus
that causes dengue. In nations plagued by dengue fever, people are urged to empty stagnant
water from old tires, trash cans, and flower pots. Governmental initiatives to decrease
mosquitoes also help to keep the disease in check but have been poorly effective.
To prevent mosquito bites, wear long pants and long sleeves. For personal protection, use
mosquito repellant sprays that contain DEET when visiting places where dengue is endemic.
Limiting exposure to mosquitoes by avoiding standing water and staying indoors two hours
after sunrise and before sunset will help. The Aedes aegypti mosquito is a daytime biter with
peak periods of biting around sunrise and sunset. It may bite at any time of the day and is
often hidden inside homes or other dwellings, especially in urban areas.
There is currently no vaccine available for dengue fever. There is a vaccine undergoing
clinical trials, but it is too early to tell if it will be safe or effective. Early results of clinical
trials show that a vaccine may be available by 2012.
3.5.10 Conclusion
Dengue fever is a disease caused by a family of viruses that are transmitted by
mosquitoes.
Symptoms such as headache, fever, exhaustion, severe joint and muscle pain, swollen
glands (lymphadenopathy), and rash. The presence (the "dengue triad") of fever, rash,
and headache (and other pains) is particularly characteristic of dengue fever.
Dengue is prevalent throughout the tropics and subtropics. Outbreaks have occurred
recently in the Caribbean, including Puerto Rico, the U.S. Virgin Islands, Cuba, and
Central America.
Because dengue fever is caused by a virus, there is no specific medicine or antibiotic
to treat it. For typical dengue fever, the treatment is purely concerned with relief of the
symptoms (symptomatic).
26
The acute phase of the illness with fever and myalgias lasts about one to two weeks.
Dengue hemorrhagic fever (DHF) is a specific syndrome that tends to affect children
under 10 years of age. It causes abdominal pain, hemorrhage (bleeding), and
circulatory collapse (shock).
The prevention of dengue fever requires control or eradication of the mosquitoes
carrying the virus that causes dengue.
There is currently no vaccine available for dengue fever.
3.6 Summary
Malaria is an infectious disease caused by a parasite, Plasmodium, which infects red blood
cells. Malaria is characterized by cycles of chills, fever, pain and sweating. Historical records
suggest malaria has infected humans since the beginning of mankind.
The disease is transmitted to humans when an infected Anopheles mosquito bites a person and
injects the malaria parasites (sporozoites) into the blood.
Sporozoites travel through the bloodstream to the liver, mature, and eventually infect the
human red blood cells.
The period between the mosquito bite and the onset of the malarial illness is usually one to
three weeks (seven to 21 days). This initial time period is highly variable as reports suggest
that the range of incubation periods may range from four days to one year.
Three main factors determine treatments:
i.
ii.
iii.
The infecting species of Plasmodium parasite,
The clinical situation of the patient (for example, adult, child, or pregnant female with
either mild or severe malaria), and
The drug susceptibility of the infecting parasites.
Malaria may pose a serious threat to a pregnant woman and her pregnancy. Malaria infection
in pregnant women may be more severe than in women who are not pregnant. Malaria poses
great danger to children especially under five year. Malaria can be prevented.
Yellow fever is a tropical disease that is spread to humans by infected mosquitoes. Many
yellow fever infections are mild, but the disease can cause severe, life-threatening illness.
Yellow fever is found only in Africa and South America. Yellow fever is preventable by
immunization. Travelers to countries with yellow fever should get the yellow fever vaccine.
Dengue fever is a disease caused by a family of viruses that are transmitted by mosquitoes.
Symptoms such as headache, fever, exhaustion, severe joint and muscle pain, swollen glands
(lymphadenopathy), and rash. The presence (the "dengue triad") of fever, rash, and headache
(and other pains) is particularly characteristic of dengue fever.
Dengue is prevalent throughout the tropics and subtropics. Because dengue fever is caused by
a virus, there is no specific medicine or antibiotic to treat it. For typical dengue fever, the
treatment is purely concerned with relief of the symptoms (symptomatic). The prevention of
dengue fever requires control or eradication of the mosquitoes carrying the virus that causes
dengue. There is currently no vaccine available for dengue fever.
27
3.7 Self-Test Questions
i)
ii)
iii)
iv)
v)
Describe causes, signs &symptoms, prevention and control of malaria.
What precautions should one take to avoid malaria?
Describe causes, signs &symptoms, prevention and control of yellow fever.
Describe what dengue fever is.
Explain the factors that contribute to dengue fever among your community
members.
vi) Describe how the infection can be controlled and prevented.
vii) List the signs and symptoms dengue fever.
28
LECTURE FOUR
TROPICAL DISEASES: TUBERCULOSIS (TB) AND CHOLERA
4.1 Introduction
Welcome to the fourth lecture in our course. In this lecture we will cover tropical diseases –
tuberculosis, and cholera. The broader objective of this lecture is to enable you to describe
tropical diseases – tuberculosis, and cholera.
4.2 Objectives
By end of this lecture you should be able to:
i) Describe what tuberculosis and cholera are.
ii) Explain the causes, symptoms and signs, diagnosis, treatment, and
prevention and control of tuberculosis and cholera.
4.3 TUBERCULOSIS (TB)
The following questions will be useful as we discuss through this section.
What is tuberculosis?
How does a person get TB?
What happens to the body when a person gets TB?
How common is TB, and who gets it?
What are the symptoms of tuberculosis?
How does a doctor diagnose tuberculosis?
Is there a vaccine against tuberculosis?
How is tuberculosis treated?
What is drug-resistant TB?
What's in the future for TB?
4.3.1 What tuberculosis is
Tuberculosis (TB) is an infectious disease caused by bacteria whose scientific name is
Mycobacterium tuberculosis. It was first isolated in 1882 by a German physician named
Robert Koch who received the Nobel Prize for this discovery.
TB most commonly affects the lungs but also can involve almost any organ of the body.
Many years ago, this disease was referred to as "consumption" because without effective
treatment, these patients often would waste away. Today, of course, tuberculosis usually can
be treated successfully with antibiotics.
There is also a group of organisms referred to as atypical tuberculosis. These involve other
types of bacteria that are in the Mycobacterium family. Often, these organisms do not cause
disease and are referred to as "colonizers" because they simply live alongside other bacteria in
our bodies without causing damage. At times, these bacteria can cause an infection that is
29
sometimes clinically like typical tuberculosis. When these atypical mycobacteria cause
infection, they are often very difficult to cure. Often, drug therapy for these organisms must
be administered for one and a half to two years and requires multiple medications.
4.3.2 Transmission of TB
A person can become infected with tuberculosis bacteria when he or she inhales minute
particles of infected sputum from the air. The bacteria get into the air when someone who has
a tuberculosis lung infection coughs, sneezes, shouts, or spits (which is common in some
cultures). People who are nearby can then possibly breathe the bacteria into their lungs.
You don't get TB by just touching the clothes or shaking the hands of someone who is
infected. Tuberculosis is spread (transmitted) primarily from person to person by breathing
infected air during close contact.
There is a form of atypical tuberculosis, however, that is transmitted by drinking
unpasteurized milk. Related bacteria, called Mycobacterium bovis, cause this form of TB.
Previously, this type of bacteria was a major cause of TB in children, but it rarely causes TB
now since most milk is pasteurized (undergoes a heating process that kills the bacteria).
4.3.3 What happens to the body when a person gets TB?
When the inhaled tuberculosis bacteria enter the lungs, they can multiply and cause a local
lung infection (pneumonia). The local lymph nodes associated with the lungs may also
become involved with the infection and usually become enlarged. The hilar lymph nodes (the
lymph nodes adjacent to the heart in the central part of the chest) are often involved.
In addition, TB can spread to other parts of the body. The body's immune (defense) system,
however, can fight off the infection and stop the bacteria from spreading. The immune system
does so ultimately by forming scar tissue around the TB bacteria and isolating it from the rest
of the body. Tuberculosis that occurs after initial exposure to the bacteria is often referred to
as primary TB. If the body is able to form scar tissue (fibrosis) around the TB bacteria, then
the infection is contained in an inactive state. Such an individual typically has no symptoms
and cannot spread TB to other people. The scar tissue and lymph nodes may eventually
harden, like stone, due to the process of calcification of the scars (deposition of calcium from
the bloodstream in the scar tissue). These scars often appear on X-rays and imaging studies
like round marbles and are referred to as a granuloma. If these scars do not show any evidence
of calcium on X-ray, they can be difficult to distinguish from cancer.
Sometimes, however, the body's immune system becomes weakened, and the TB bacteria
break through the scar tissue and can cause active disease, referred to as reactivation
tuberculosis or secondary TB. For example, the immune system can be weakened by old age,
the development of another infection or a cancer, or certain medications such as cortisone,
anticancer drugs, or certain medications used to treat arthritis or inflammatory bowel disease.
The breakthrough of bacteria can result in a recurrence of the pneumonia and a spread of TB
to other locations in the body. The kidneys, bone, and lining of the brain and spinal cord
(meninges) are the most common sites affected by the spread of TB beyond the lungs.
30
4.3.4 Distribution and vulnerability for TB
Over 8 million new cases of TB occur each year worldwide. Anyone can get TB, but certain
people are at higher risk, including
people who live with individuals who have an active TB infection,
poor or homeless people,
foreign-born people from countries that have a high prevalence of TB,
nursing-home residents and prison inmates,
alcoholics and intravenous drug users,
people with diabetes, certain cancers, and HIV infection (the AIDS virus),
Health-care workers.
There is no strong evidence for a genetically determined (inherited) susceptibility for TB.
4.3.5 Signs and symptoms of tuberculosis
As previously mentioned, TB infection usually occurs initially in the upper part (lobe) of the
lungs. The body's immune system, however, can stop the bacteria from continuing to
reproduce. Thus, the immune system can make the lung infection inactive (dormant). On the
other hand, if the body's immune system cannot contain the TB bacteria, the bacteria will
reproduce (become active or reactivate) in the lungs and spread elsewhere in the body.
It may take many months from the time the infection initially gets into the lungs until
symptoms develop. The usual symptoms that occur with an active TB infection are a
generalized tiredness or weakness, weight loss, fever, and night sweats. If the infection in the
lung worsens, then further symptoms can include coughing, chest pain, coughing up of
sputum (material from the lungs) and/or blood, and shortness of breath. If the infection
spreads beyond the lungs, the symptoms will depend upon the organs involved.
4.3.6 Diagnosis tuberculosis
TB can be diagnosed in several different ways, including chest X-rays, analysis of sputum,
and skin tests. Sometimes, the chest X-rays can reveal evidence of active tuberculosis
pneumonia.
Other times, the X-rays may show scarring (fibrosis) or hardening (calcification) in the lungs,
suggesting that the TB is contained and inactive. Examination of the sputum on a slide
(smear) under the microscope can show the presence of the tuberculosis-like bacteria.
Bacteria of the Mycobacterium family, including atypical mycobacteria, stain positive with
special dyes and are referred to as acid-fast bacteria (AFB).
A sample of the sputum also is usually taken and grown (cultured) in special incubators so
that the tuberculosis bacteria can subsequently be identified as tuberculosis or atypical
tuberculosis.
Several types of skin tests are used to screen for TB infection. These so-called tuberculin skin
tests include the Tine test and the Mantoux test, also known as the PPD (purified protein
derivative) test. In each of these tests, a small amount of purified extract from dead
31
tuberculosis bacteria is injected under the skin. If a person is not infected with TB, then no
reaction will occur at the site of the injection (a negative skin test).
If a person is infected with tuberculosis, however, a raised and reddened area will occur
around the site of the test injection. This reaction, a positive skin test, occurs about 48-72
hours after the injection. When only the skin test is positive, or evidence of prior TB is present
on chest X-rays, the disease is referred to as "latent tuberculosis." This contrasts with active
TB as described above, under symptoms.
If the infection with tuberculosis has occurred recently, however, the skin test can be falsely
negative. The reason for a false-negative test with a recent infection is that it usually takes two
to 10 weeks after the time of infection with tuberculosis before the skin test becomes positive.
The skin test can also be falsely negative if a person's immune system is weakened or
deficient due to another illness such as AIDS or cancer, or while taking medications that can
suppress the immune response, such as cortisone or anticancer drugs.
Remember, however, that the TB skin test cannot determine whether the disease is active or
not. This determination requires the chest X-rays and/or sputum analysis (smear and culture)
in the laboratory. The organism can take up to six weeks to grow in culture in the
microbiology lab. A special test to diagnose TB called the PCR (polymerase chain reaction)
detects the genetic material of the bacteria. This test is extremely sensitive (it detects minute
amounts of the bacteria) and specific (it detects only the TB bacteria). One can usually get
results from the PCR test within a few days.
4.3.7 Tuberculosis vaccine
Bacille Calmette Guérin, also known as BCG, is a vaccine given throughout many parts of the
world. It is derived from an atypical Mycobacterium but offers some protection from
developing active tuberculosis, especially in infants and children. This vaccination is believed
to be important in parts of the world where TB is quite common. This is not the case in the
United States. When BCG has been administered, future PPD and Tine skin tests remain
positive and can cause some confusion when trying to diagnose TB. It is also important to
realize that even with a BCG vaccine in childhood, tuberculosis can still occur in an adult
exposed to the tuberculosis bacteria, which calls into question the real utility and effectiveness
of this vaccination.
A new blood test is now available that can help distinguish between a prior BCG vaccine and
a positive PPD due to TB infection. This test involves mixing the patient's blood with
substances that produce a TB-like immune response. After a period of time, the immune cells,
if infected with TB, produce interferon-gamma, a protein produced by the body to defend
against an infection. This test, like most, is not perfect, but with the proper clinical
information can help distinguish a real TB infection from a positive reaction on the test due to
a prior BCG vaccine.
4.3.8 Treatment of tuberculosis
A person with a positive skin test, a normal chest X-ray, and no symptoms most likely has
only a few TB germs in an inactive state and is not contagious. Nevertheless, treatment with
an antibiotic may be recommended for this person to prevent the TB from turning into an
active infection. The antibiotic used for this purpose is called isoniazid (INH). If taken for six
32
to 12 months, it will prevent the TB from becoming active in the future. In fact, if a person
with a positive skin test does not take INH, there is a 5%-10% lifelong risk that the TB will
become active.
Taking isoniazid can be inadvisable (contraindicated) during pregnancy or for those suffering
from alcoholism or liver disease. Also, isoniazid can have side effects. The side effects occur
infrequently, but a rash can develop, and the individual can feel tired or irritable.
Liver damage from isoniazid is a rare occurrence and typically reverses once the drug is
stopped. Very rarely, however, especially in older people, the liver damage (INH hepatitis)
can even be fatal. It is important therefore, for the doctor to monitor a patient's liver by
periodically ordering blood tests called "liver function tests" during the course of INH
therapy. Another side effect of INH is a decreased sensation in the extremities referred to as a
peripheral neuropathy. This can be avoided by taking vitamin B6 (pyridoxine), and this is
often prescribed along with INH.
A person with a positive skin test along with an abnormal chest X-ray and sputum evidencing
TB bacteria has active TB and is contagious. As already mentioned, active TB usually is
accompanied by symptoms, such as a cough, fever, weight loss, and fatigue.
Active TB is treated with a combination of medications along with isoniazid. Rifampin
(Rifadin), ethambutol (Myambutol), and pyrazinamide are the drugs commonly used to treat
active TB in conjunction with isoniazid (INH). Four drugs are often taken for the first two
months of therapy to help kill any potentially resistant strains of bacteria. Then the number is
usually reduced to two drugs for the remainder of the treatment based on drug sensitivity
testing that is usually available by this time in the course.
Streptomycin, a drug that is given by injection, may be used as well, particularly when the
disease is extensive and/or the patients do not take their oral medications reliably (termed
"poor compliance"). Treatment usually lasts for many months and sometimes for years.
Successful treatment of TB is dependent largely on the compliance of the patient. Indeed, the
failure of a patient to take the medications as prescribed is the most important cause of failure
to cure the TB infection. In some locations, the health department demands direct monitoring
of patient compliance with therapy.
Surgery on the lungs may be indicated to help cure TB when medication has failed, but in this
day and age, surgery for TB is unusual. Treatment with appropriate antibiotics will usually
cure the TB. Without treatment, however, tuberculosis can be a lethal infection. Therefore,
early diagnosis is important.
4.3.9 Drug-resistant TB
Drug-resistant TB (TB that does not respond to drug treatment) has become a very serious
problem in recent years in certain populations. For example, INH-resistant TB is seen among
patients from Southeast Asia. The presence of INH-like substances in the cough syrups in that
part of the world may play a role in causing the INH resistance. Drug-resistant cases are also
often seen in prison populations. However, the major reason for the development of resistance
is poorly managed TB care.
33
This can result from poor patient compliance, inappropriate dosing or prescribing of
medication, poorly formulated medications, and/or an inadequate supply of medication.
Multidrug-resistant tuberculosis (MDR-TB) refers to organisms that are resistant to at least
two of the first-line drugs, INH and Rifampin. More recently, extensively (extremely) drug
resistant tuberculosis (XDR-TB) has emerged. These bacteria are also resistant to three or
more of the second-line treatment drugs.
XDR-TB is seen throughout the world but is most frequently seen in the countries of the
former Soviet Union and Asia.
Preventing XDR-TB from spreading is essential. The World Health Organization (WHO)
recommends improving basic TB care to prevent emergence of resistance and the
development of proper laboratories for detection of resistant cases. When drug-resistant cases
are found, prompt, appropriate treatment is required. This will prevent further transmission.
Collaboration of HIV and TB care will also help limit the spread of tuberculosis, both
sensitive and resistant strains.
4.3.10 The future for TB.
Conceivably, TB could have been eliminated by effective treatment, vaccinations, and publichealth measures by the year 2000. However, the emergence of HIV changed the whole
picture.
Because of HIV, a tremendous increase in the frequency (incidence) of TB occurred in the
'80s and throughout the '90s. This increase in TB happened because suppression of the body's
immune (defense) system by HIV allowed TB to occur as a so-called opportunistic infection.
With the increasing HIV epidemic in Africa, serious concerns are being raised about the
development of MDR-TB and XDR-TB in this population. Hopefully, control of HIV in the
future will check this resurgence of tuberculosis.
4.3.11 Conclusion
Tuberculosis (TB) is an infection, primarily in the lungs (a pneumonia), caused by
bacteria called Mycobacterium tuberculosis. It is spread usually from person to person
by breathing infected air during close contact.
TB can remain in an inactive (dormant) state for years without causing symptoms or
spreading to other people.
When the immune system of a patient with dormant TB is weakened, the TB can
become active (reactivate) and cause infection in the lungs or other parts of the body.
The risk factors for acquiring TB include close-contact situations, alcohol and IV drug
abuse, and certain diseases (for example, diabetes, cancer, and HIV) and occupations
(for example, health-care workers).
The most common symptoms of TB are fatigue, fever, weight loss, coughing, and
night sweats.
The diagnosis of TB involves skin tests, chest X-rays, sputum analysis (smear and
culture), and PCR tests to detect the genetic material of the causative bacteria.
Inactive tuberculosis may be treated with an antibiotic, isoniazid (INH), to prevent the
TB infection from becoming active.
34
Active TB is treated, usually successfully, with INH in combination with one or more
of several drugs, including rifampin, ethambutol, pyrazinamide, and streptomycin.
Drug-resistant TB is a serious, as yet unsolved, public-health problem, especially in
Southeast Asia, the countries of the former Soviet Union, Africa, and in prison
populations. Poor patient compliance, lack of detection of resistant strains, and
unavailable therapy are key reasons for the development of drug-resistant TB.
The occurrence of HIV has been responsible for an increased frequency of
tuberculosis. Control of HIV in the future, however, should substantially decrease the
frequency of TB.
4.4 CHOLERA
Get answers to the following questions as you read through this section.
What is cholera?
What are cholera symptoms?
How does a person get cholera?
What should travelers do to avoid getting cholera?
Is a vaccine available to prevent cholera?
Can cholera be treated?
4.4.1 Meaning of cholera.
Cholera is an acute, diarrheal illness caused by infection of the intestine with the bacterium
Vibrio cholerae. The infection is often mild or without symptoms, but sometimes can be
severe.
4.4.2 Signs and symptoms of cholera
Approximately 1 in 20 infected persons has severe disease characterized by:
profuse watery diarrhea,
vomiting, and
Leg cramps.
In these persons, rapid loss of body fluids leads to dehydration and shock. Without treatment,
death can occur within hours.
4.4.3 Transmission of cholera
A person may get cholera by drinking water or eating food contaminated with the Vibrio
cholerae. In an epidemic, the source of the contamination is usually the feces (stool) of an
infected person. The disease can spread rapidly in areas with inadequate treatment of sewage
and drinking water.
The cholera bacterium may also live in the environment in brackish rivers and coastal waters.
Shellfish eaten raw have been a source of cholera, and a few persons in the United States have
contracted cholera after eating raw or undercooked shellfish from the Gulf of Mexico. The
disease is not likely to spread directly from one person to another; therefore, casual contact
with an infected person is not a risk for becoming ill.
35
4.4.4 Prevention of cholera
All travelers to areas where cholera has occurred should observe the following
recommendations:
Drink only water that you have boiled or treated with chlorine or iodine. Other safe
beverages include tea and coffee made with boiled water and carbonated bottled
beverages with no ice.
Eat only foods that have been thoroughly cooked and are still hot, or fruit that you
have peeled yourself.
Avoid undercooked or raw fish or shellfish, including ceviche.
Make sure all vegetables are cooked, avoid salads.
Avoid foods and beverages from street vendors.
A simple rule of thumb is:
"Boil it, cook it, peel it, or forget it."
4.4.5 Cholera Vaccine
Two recently developed vaccines for cholera are licensed. Both vaccines appear to provide a
somewhat better immunity and fewer side-effects than the previously available vaccine.
However, neither of these two vaccines is recommended for travelers.
4.4.6 Treatment of cholera
Cholera can be simply and successfully treated by immediate replacement of the fluid and
salts lost through diarrhea. Patients can be treated with oral rehydration solution, a
prepackaged mixture of sugar and salts to be mixed with water and drunk in large amounts.
This solution is used throughout the world to treat diarrhea. Severe cases also require
intravenous fluid replacement. With prompt rehydration, fewer than 1% of cholera patients
die.
Antibiotics shorten the course and diminish the severity of the illness, but they are not as
important as rehydration. Persons who develop severe diarrhea and vomiting in countries
where cholera occurs should seek medical attention promptly.
4.6 Summary
Tuberculosis (TB) is an infection, primarily in the lungs (a pneumonia), caused by bacteria
called Mycobacterium tuberculosis. It is spread usually from person to person by breathing
infected air during close contact. TB can remain in an inactive (dormant) state for years
without causing symptoms or spreading to other people. When the immune system of a
patient with dormant TB is weakened, the TB can become active (reactivate) and cause
infection in the lungs or other parts of the body.
The risk factors for acquiring TB include close-contact situations, alcohol and IV drug abuse,
and certain diseases (for example, diabetes, cancer, and HIV) and occupations (for example,
health-care workers). The most common symptoms of TB are fatigue, fever, weight loss,
coughing, and night sweats. The diagnosis of TB involves skin tests, chest X-rays, sputum
36
analysis (smear and culture), and PCR tests to detect the genetic material of the causative
bacteria. Inactive tuberculosis may be treated with an antibiotic, isoniazid (INH), to prevent
the TB infection from becoming active.
Active TB is treated, usually successfully, with INH in combination with one or more of
several drugs, including rifampin, ethambutol, pyrazinamide, and streptomycin. Drugresistant TB is a serious, as yet unsolved, public-health problem, especially in Southeast Asia,
the countries of the former Soviet Union, Africa, and in prison populations. Poor patient
compliance, lack of detection of resistant strains, and unavailable therapy are key reasons for
the development of drug-resistant TB. The occurrence of HIV has been responsible for an
increased frequency of tuberculosis. Control of HIV in the future, however, should
substantially decrease the frequency of TB.
Cholera is an acute, diarrheal illness caused by infection of the intestine with the bacterium
Vibrio cholerae. The infection is often mild or without symptoms, but sometimes can be
severe disease characterized by: profuse watery diarrhea, vomiting, and Leg cramps. In these
persons, rapid loss of body fluids leads to dehydration and shock. Without treatment, death
can occur within hours.
4.7 Self-Test Questions
i) Describe the meaning of the following;
-
tuberculosis,
cholera
ii) Explain the causes, symptoms and signs, diagnosis, treatment, and prevention
and control of tuberculosis, and cholera.
37
LECTURE FIVE
TROPICAL DISEASES: TRYPANOSOMIASIS AND LEISHMANIASIS
5.1 Introduction
Welcome to the fifth lecture in our course. In this lecture we will cover tropical diseases –
Trypanosomiasis and leishmaniasis. The broader objective of this lecture is to enable you to
describe tropical diseases - trypanosomiasis and leishmaniasis.
5.2 Objectives
By end of this lecture you should be able to:
i) Describe what trypanosomiasis and leishmaniasis are.
ii) Explain the causes, symptoms and signs, diagnosis, treatment, and
prevention and control of trypanosomiasis and leishmaniasis.
5.4 TRYPANOSOMIASIS (SLEEPING SICKNESS)
5.4.1 Meaning of Trypanosomiasis
Trypanosomiasis refers to three types of infections caused by protozoa and spread to humans
through tsetseflies bites. There are two kinds of African trypanosomiasis, East African and
West African. Both of these varieties also are known as sleeping sickness. The disease can
affect people living on the African continent south of the Sahara Desert. American
trypanosomiasis also is called Chagas disease. It occurs only on the American continents,
from Mexico to Argentina.
5.4.2 Causes of Trypanosomiasis
The bite of an infected tsetse fly usually transmits the organisms that cause the African forms
of trypanosomiasis. These flies live in the countryside in Africa, especially in bushes and
thick vegetation near rivers and lakes. Tsetse flies infected with the protozoan Trypanosoma
brucei rhodesiense spread East African trypanosomiasis, the most severe form of the disease,
to humans. The West African variety comes from a fly infected with Trypanosoma brucei
gambiense.
Reduviid bugs (also called assassin, cone-nose, or kissing bugs) carry the Trypanosoma cruzi
protozoa that cause the American variety of trypanosomiasis, or Chagas disease, named for
the Brazilian doctor who discovered it. These bugs hide during the day in the cracks in mud
and adobe homes. At night they crawl across sleeping people and bite them, usually on the
face but sometimes on the arms, legs, or trunk. They also leave behind their feces * , which
contain the protozoa. Without knowing it, people can rub
38
5.4.3 Distribution of Trypanosomiasis
Trypanosomiasis can infect people of very age and race. The disease affects thousands of
people around the world. The World Health Organization estimates that as many as 500,000
people could have African trypanosomiasis, but because of poor monitoring most of these
cases are not reported. Between 16 million and 18 million people in the Americas currently
have Chagas disease. Approximately 50,000 may die from the disease each year.
5.4.4 Is Trypanosomiasis Contagious?
People cannot catch any form of trypanosomiasis in the same way that they catch a cold or the
flu from other people. Only the tsetse fly spreads the African varieties, and the reduviid bug
spreads Chagas disease. Rarely, a mother infected with the West African variety of
trypanosomiasis or with Chagas disease can pass the illness to her unborn child. People who
receive a transfusion of blood or an organ transplant from an infected person also may
contract the disease; this form of transmission tends to happen more often with Chagas
disease than with the African types.
5.4. 5 Signs and Symptoms of the Disease
People who contract the African varieties of trypanosomiasis may start sleeping more, though
this usually does not happen until the later stages of the disease. Sleeping sickness may start
with the appearance of a sore called a chancre (SHANG-ker) at the spot where the person
received the tsetse fly bite.
Later symptoms include fever, extreme tiredness, severe headaches, rashes, itching, joint pain,
and swelling of the hands and feet. The lymph nodes on the back of the neck may become
swollen as well. These signs typically appear 2 to 4 weeks after infection with East African
trypanosomiasis.
Other symptoms can follow quickly, as the protozoa cross the blood-brain barrier and start
affecting a patient's mental functions. The later stages of sleeping sickness may bring mental
confusion, changes in personality, problems with walking and talking, weight loss, and
seizures. The spleen and liver may become enlarged.
Sleeping sickness gets its name from the later part of the disease, when the sick person has
nighttime insomnia but sleeps for long periods during the day. If the person does not receive
treatment, the heart muscles may become inflamed or weakened, causing death from heart
failure.
The early symptoms in West African trypanosomiasis are similar but may take longer to
appear. Months or years may pass before an infected person becomes sick, and the disease
develops more slowly, though it still can cause death if it is left untreated. The gap between
infection and the start of symptoms can make this form of sleeping sickness difficult to
diagnose.
39
5.4.6 Chagas disease
The first sign of Chagas disease may show up a few hours after infection, when a raised red
spot called a chagoma appears at the site of the insect bite. Most people have no other
symptoms during the early, or acute, phase of the disease, which begins a few weeks later.
People who experience symptoms may have fevers, rashes, extreme tiredness, vomiting, loss
of appetite, or swollen lymph nodes. The side of the face where the infected feces were
rubbed into an eye or a bug bite may swell. In most people these symptoms usually disappear
within 4 to 8 weeks without causing problems, but infants can die in this early stage from
brain swelling.
About 10 to 20 years after this first phase, approximately one-third of infected people can
show symptoms of the chronic phase of Chagas disease. They may become constipated and
experience trouble swallowing. The heart may become enlarged, and patients may have
altered heart rhythms or heart failure leading to death.
5.4.7 Diagnosis Trypanosomiasis
To diagnose sleeping sickness or Chagas disease, a doctor will order blood tests to look for
protozoa or antibodies to the organism. In cases where the doctor suspects sleeping sickness, a
sample drawn from fluid surrounding the brain and spinal cord or tissue from swollen lymph
nodes may be examined for evidence of the disease. If a patient has a suspicious-looking skin
lesion, a biopsy will be performed to test for Trypanosoma cruzi protozoa.
5.4.8 Treatment of Trypanosomiasis.
There are medications available to treat all types of the disease. Doctors recommend that
people with trypanosomiasis receive treatment as soon as possible. Treatment is given in a
hospital. After leaving the hospital, patients typically are watched closely by a doctor for at
least 2 years, to see whether they show any signs that they still have the infection.
5.4.9 Reasons why the disease is common in tropics
Why are these diseases common to the tropics? It is because the hot and often rainy climate
makes the tropics an ideal breeding ground for insects. Greenpeace, among other
organizations dedicated to protecting the environment, has warned that global warming could
create new breeding grounds for insects throughout the world. At the same time, rising
temperatures could raise insect reproductive rates, increasing their numbers.
5.4.10 Consequences of Trypanosomiasis
East African sleeping sickness can move through the body quickly, progressing in just weeks
or months to the most serious phase of illness. West African sleeping sickness takes longer to
develop. People may not reach the critical phase for months or even years. People who do not
receive treatment for African trypanosomiasis can die from heart failure, and those who wait
to start treatment may have permanent brain damage. Long-term complications of Chagas
disease, which may not appear for 20 or more years after infection, include damage to the
digestive and nervous systems, heart problems, and sudden death.
40
5.4.11 Prevention of Trypanosomiasis
There is no vaccine or medication that can prevent any form of the disease, so it is wise for
people who travel in areas where the disease is common to take precautions.
This includes:
•
•
•
•
•
•
Wearing clothes of thick material, with long sleeves and long pants.
Neutral colors, such as tan, are best because tsetse flies are attracted to dark and bright
colors.
Sleep under net and
Avoid riding in the backs of open trucks, because dust from moving vehicles attracts
the flies.
It is also advisable not to walk through brush.
In areas where Chagas disease is found, it is a good idea for people to avoid sleeping
in mud, adobe, or thatch houses; to sleep under netting; and to use insect repellent.
5.5 LEISHMANIASIS
5.5.1 Meaning of leishmaniasis
Leishmaniasis is a parasitic disease spread by the bite of infected sand flies. There are several
different forms of leishmaniasis. The most common forms are:
•
•
Cutaneous leishmaniasis, which causes skin sores, and
Visceral leishmaniasis, which affects some of the internal organs of the body (for
example, spleen, liver, bone marrow).
5.5.2 Signs and symptoms of cutaneous leishmaniasis
People who have cutaneous leishmaniasis have one or more sores on their skin. The sores can
change in size and appearance over time. They often end up looking somewhat like a volcano,
with a raised edge and central crater. Some sores are covered by a scab. The sores can be
painless or painful. Some people have swollen glands near the sores (for example, under the
arm if the sores are on the arm or hand).
5.5.3 Signs and symptoms of visceral leishmaniasis
People who have visceral leishmaniasis usually have fever, weight loss, and an enlarged
spleen and liver (usually the spleen is bigger than the liver). Some patients have swollen
glands. Certain blood tests are abnormal. For example, patients usually have low blood
counts, including a low red blood cell count (anemia), low white blood cell count, and low
platelet count.
5.5.4 Distribution of leishmaniasis.
The number of new cases of cutaneous leishmaniasis each year in the world is thought to be
about 1.5 million. The number of new cases of visceral leishmaniasis is thought to be about
500,000.
41
Leishmaniasis is found in parts of about 88 countries. Approximately 350 million people live
in these areas. Most of the affected countries are in the tropics and subtropics. The settings in
which leishmaniasis is found range from rain forests in Central and South America to deserts
in West Asia. More than 90% of the world's cases of visceral leishmaniasis are in India,
Bangladesh, Nepal, Sudan, and Brazil.
Leishmaniasis is found in some parts of the following areas:
in Mexico, Central America, and South America—from northern Argentina to Texas
(not in Uruguay, Chile, or Canada)
southern Europe (leishmaniasis is not common in travelers to southern Europe)
Asia (not Southeast Asia)
the Middle East
Africa (particularly East and North Africa, with some cases elsewhere)
Leishmaniasis is not found in Australia or Oceania (that is, islands in the Pacific, including
Melanesia, Micronesia, and Polynesia).
5.5.5 Transmission of leishmaniasis
Leishmaniasis is spread by the bite of some types of phlebotomine sand flies. Sand flies
become infected by biting an infected animal (for example, a rodent or dog) or person. Since
sand flies do not make noise when they fly, people may not realize they are present.
Sand flies are very small and may be hard to see; they are only about one-third the size of
typical mosquitoes. Sand flies usually are most active in twilight, evening, and night-time
hours (from dusk to dawn).
Sand flies are less active during the hottest time of the day. However, they will bite if they are
disturbed, such as when a person brushes up against the trunk of a tree where sand flies are
resting. Rarely, leishmaniasis is spread from a pregnant woman to her baby. Leishmaniasis
also can be spread by blood transfusions or contaminated needles.
5.5.6 Who is at risk for leishmaniasis?
People of all ages are at risk for leishmaniasis if they live or travel where leishmaniasis is
found. Leishmaniasis usually is more common in rural than urban areas; but it is found in the
outskirts of some cities.
The risk for leishmaniasis is highest from dusk to dawn because this is when sand flies are the
most active. All it takes to get infected is to be bitten by one infected sand fly. This is more
likely to happen the more people are bitten, that is, the more time they spend outside in rural
areas from dusk to dawn.
5.5.7 Recovery from infection
People with cutaneous leishmaniasis usually develop skin sores within a few weeks
(sometimes as long as months) of when they were bitten.
42
People with visceral leishmaniasis usually become sick within several months (rarely as long
as years) of when they were bitten.
5.5.8 How serious leishmaniasis can be if not treated
Yes, it can be. The skin sores of cutaneous leishmaniasis will heal on their own, but this can
take months or even years. The sores can leave ugly scars. If not treated, infection that started
in the skin rarely spreads to the nose or mouth and causes sores there (mucosal leishmaniasis).
This can happen with some of the types of the parasite found in Central and South America.
Mucosal leishmaniasis might not be noticed until years after the original skin sores healed.
The best way to prevent mucosal leishmaniasis is to treat the cutaneous infection before it
spreads.
If not treated, visceral leishmaniasis can cause death.
5.5.9 Diagnosis of leishmaniasis
The first step is to find out if you have traveled to a part of the world where leishmaniasis is
found. Your health care provider will ask you about any signs or symptoms of leishmaniasis
you may have, such as skin sores that have not healed.
If you have skin sores, your health care provider will likely want to take some samples
directly from the sores. These samples can be examined for the parasite under a microscope,
in cultures, and through other means. A blood test for detecting antibody (immune response)
to the parasite can be helpful, particularly for cases of visceral leishmaniasis. However, tests
to look for the parasite itself should also be done.
Diagnosing leishmaniasis can be difficult. Sometimes the laboratory tests are negative even if
a person has leishmaniasis.
5.5.10 Treatment leishmaniasis
Most people who have cutaneous leishmaniasis do not need to be hospitalized during their
treatment.
5.5.11 Prevention of leishmaniasis
The best way for travelers to prevent leishmaniasis is by protecting themselves from sand fly
bites.
Vaccines and drugs for preventing infection are not yet available. To decrease their risk of
being bitten, travelers should:
Stay in well-screened or air-conditioned areas as much as possible. Avoid outdoor
activities, especially from dusk to dawn, when sand flies are the most active.
When outside, wear long-sleeved shirts, long pants, and socks. Tuck your shirt into
your pants.
43
Apply insect repellent on uncovered skin and under the ends of sleeves and pant legs.
Follow the instructions on the label of the repellent. The most effective repellents are
those that contain the chemical DEET (N,N-diethylmetatoluamide). The concentration
of DEET varies among repellents. Repellents with DEET concentrations of 30%-35%
are quite effective, and the effect should last about 4 hours. Lower concentrations
should be used for children (no more than 10% DEET). Repellents with DEET should
be used sparingly on children from 2 to 6 years old and not at all on children less than
2 years old.
Spray clothing with permethrin-containing insecticides. The insecticide should be
reapplied after every five washings.
Spray living and sleeping areas with an insecticide to kill insects.
If you are not sleeping in an area that is well screened or air-conditioned, use a bed net
and tuck it under your mattress. If possible, use a bed net that has been soaked in or
sprayed with permethrin. The permethrin will be effective for several months if the
bed net is not washed. Keep in mind that sand flies are much smaller than mosquitoes
and therefore can get through smaller holes. Fine-mesh netting (at least 18 holes to the
inch; some sources say even finer) is needed for an effective barrier against sand flies.
This is particularly important if the bed net has not been treated with permethrin.
However, it may be uncomfortable to sleep under such a closely woven bed net when
it is hot.
5.5.11 Reinfection
Yes. Some people have had cutaneous leishmaniasis more than once. Therefore, you should
follow the preventive measures listed above whenever you are in an area where leishmaniasis
is found.
5.5.11Conclusion
Leishmaniasis is a disease caused by the bite of an infected sand fly. The most common types
of leishmania infection are cutaneous and visceral leishmaniasis. Leishmaniasis is found
mainly in the subtropics and tropics. Symptoms and signs of cutaneous leishmaniasis include
skin sores with a raised edge and central crater, while those with visceral leishmaniasis
usually have fever, weight loss, and an enlarged liver and spleen.
5.5 Summary
Lymphatic filariasis is a disease of the tropics. It is caused by infection with any of several
rounds, thread-like parasitic worms. The most common is infection with a parasite that lives
in the lymph system. This is called lymphatic filariasis. The parasite is spread from person to
person by infected mosquitoes. Long-term exposure and repeated infections can cause severe
damage to the lymph system and serious, debilitating complications. Prevention centers on
controlling mosquito populations in communities and avoiding mosquito bites.
Trypanosomiasis refers to three types of infections caused by protozoa and spread to humans
through insect bites. There are two kinds of African trypanosomiasis, East African and West
African. Both of these varieties also are known as sleeping sickness. The bite of an infected
tsetse fly usually transmits the organisms that cause the African forms of trypanosomiasis.
Sign and symptoms include sleeping more, appearance of a sore, fever, extreme tiredness,
severe headaches, rashes, itching, joint pain, and swelling of the hands and feet etc.
44
Leishmaniasis is a disease caused by the bite of an infected sand fly. The most common types
of leishmania infection are cutaneous and visceral leishmaniasis. Leishmaniasis is found
mainly in the subtropics and tropics. Symptoms and signs of cutaneous leishmaniasis include
skin sores with a raised edge and central crater, while those with visceral leishmaniasis
usually have fever, weight loss, and an enlarged liver and spleen
5.5 Self-Test Questions
i) What are the following:
-
filariasis,
trypanosomiasis and
leishmaniasis
ii) Explain the causes, symptoms and signs, diagnosis, treatment, and prevention
and control of filariasis, trypanosomiasis and leishmaniasis.
45
LECTURE SIX
TROPICAL DISEASES: AMOEBIC, BACILLARY DYSENTERY AND TETANUS.
6.1 Introduction
Welcome to the seventh lecture in our course. In this lecture we will cover tropical diseases –
Tetanus, Amoeba and Bacillary Dysentry. The broader objective of this lecture is to enable
you to describe tropical diseases –Tetanus, Amoeba and Bacillary Dysentry.
6.2 Objectives
By end of this lecture you should be able to
i) Describe what tetanus, amoebic and bacillary dysentery are.
ii) Explain the causes,transmission, symptoms and control of Tetanus, Amoeba
and Bacillary Dysentry
6.3 AMOEBIC DYSENTERY
Amebic dysentery: Ameba-caused bacterial bowel infection and ulceration. More detailed
information about the symptoms, causes, and treatments of Amebic dysentery is available
below.
6.3.1 What amoebic dysentery is
Amoebic dysentery is an intestinal illness associated with stomach pain, bloody stools,
and fever. This condition can be treated.
6.3.2 Causes
Amoebic dysentery is caused by a parasite called Entamoeba histolytica. You may
develop amoebic dysentery if you:
i.
ii.
iii.
Put something in your mouth that has touched the stool of a person infected
with E histolytica
Swallow water or food that has been contaminated with E histolytica
Touch cysts (eggs) from E histolytica -contaminated surfaces and bring them
to your mouth
6.3.3 Risk Factors
A risk factor is something that increases your chance of getting a disease or condition. The
following risk factors increase your chance of developing amoebic dysentery. If you have any
of these risk factors, tell your doctor:
46
Living in or traveling to developing countries, places that have poor sanitary
conditions, or tropical or subtropical areas
Living in institutions
Having anal-sexual intercourse
6.3.5 Symptoms of Amebic dysentery
The list of signs and symptoms for Amebic dysentery includes the symptoms listed below:
No symptoms - some people are symptomless carriers
Onset may be sudden or over years
Mild early symptoms - when onset is slow
Weight loss
Anemia
Indigestion
Blood in stool
Dehydration
Symptoms of an abscess caused by amebiasis:
o High fever
o Chills
o Pain
Loose stools
Nausea
Stomach pain
Stomach cramping
6.3.6 Diagnosis
Your doctor will ask about your symptoms and medical history, and perform a physical exam.
Tests may include the following:
Stool samples
Blood tests
6.3.7 Treatment
Talk with your doctor about the best treatment plan for you. Treatment options include the
following:
6.3.7.1 Medications
Several antibiotics are available to treat amoebic dysentery. Flagyl is also used.
6.3.7.2 Prevention
To help reduce your chances of getting amoebic dysentery, take the following steps when
traveling to a country that has poor sanitary conditions:
Drink only bottled water or water that has been boiled for at least one minute
47
Do not eat fresh fruit or vegetables that you do not peel yourself
Do not eat or drink unpasteurized milk, cheese, or dairy products
Do not eat or drink anything sold by street vendors
6.4 BACILLARY DYSENTERY
6.4.1 What bacillary dysentery is
Bacillary dysentery is caused by certain nonmotile bacteria of the genus Shigella. This
form of dysentery is also most prevalent in unhygienic areas of the Tropics, but,
because it is easily spread, sporadic outbreaks are common in all parts of the world.
This dysentery is usually self-limiting and rarely manifests the more severe organ
involvements characteristic of amoebic dysentery.
6.4.2 Transmissiom
Bacillary dysentery is spread by contaminated water, milk, and food.
Feces from active cases and those from healthy carriers as well contain immense
numbers of the disease-producing bacteria.
Flies carry the bacteria on their feet or in their saliva and feces and deposit them on
food; ants are also believed to spread the disease.
6.4.3 Treatment
In the treatment of bacillary dysentery, proper replacement of fluid is important.
Sulfonamides, tetracycline, and streptomycin were effective in curing acute cases until
drug-resistant strains emerged.
Chloramphenicol is sometimes used to treat these strains.
Quinolones such as norfloxacin and ciprofloxacin are also effective against Shigella
infection.
6.5 TETANUS.
6.5.1 What Tetanus is
Tetanus is an infectious disease caused by contamination of wounds from bacteria that
live in the soil.
The causative bacterium Clostridium tetani is a hardy organism capable of living
many years in the soil in a form called a spore.
The bacterium was first isolated in 1899 by S. Kitasato while he was working with R.
Koch in Germany. Kitasato also found the toxin responsible for tetanus and developed
the first protective vaccine against the disease.
Tetanus occurs when a wound becomes contaminated with bacteria spores. Infection
follows when spores become activated and develop into gram-positive bacteria that
multiply and produce a very powerful toxin (poison) that affects the muscles.
Tetanus spores are found throughout the environment, usually in soil, dust, and animal
waste.
The usual locations for the bacteria to enter your body are puncture wounds, such as
those caused by rusty nails, splinters or insect bite. Burns, any break in the skin, and
48
IV drug access sites are also potential entryways for the bacteria Tetanus is acquired
through contact with environment; it is not transmitted from person to person.
Tetanus results in severe, uncontrollable muscle spasms. The jaw is "locked" by
muscle spasms, causing the disease to sometimes be called "lockjaw." In severe cases,
the muscles used to breathe can spasm, causing a lack of oxygen to the brain and other
organs that may possibly lead to death.
The disease in humans is the result of infection of a wound with the spores of the
bacteria Clostridium tetani. These bacteria produce the toxin (poison) tetanospasmin,
which is responsible for causing tetanus.
Tetanospasmin binds to motor nerves that control muscles, enters the axons (filaments
that extend from nerve cells), and travels in the axon until it reaches the body of the
motor nerve in the spinal cord or brainstem (a process termed retrograde intraneuronal
transport). Then the toxin migrates into the synapse (small space between nerve cells
critical for transmission of signals among nerve cells) where it binds to presynaptic
nerve terminals and inhibits or stops the release of certain inhibitory neurotransmitters
(glycine and gamma-aminobutyric acid).
Because the motor nerve has no inhibitory signals from other nerves, the chemical
signal to the motor nerve of the muscle intensifies, causing the muscle to tighten up in
a huge continuous contraction or spasm. If tetanospasmin reaches the bloodstream or
lymphatic vessels from the wound site, it can be deposited in many different
presynaptic terminals resulting in the same effect on other muscles.
In developing countries of Africa, Asia, and South America, tetanus is far more common. The
annual worldwide incidence is between 500,000-1 million cases. The majority of new cases
worldwide are in neonates in Third World countries.
The disease can show four possible types:
o Generalized tetanus can affect all skeletal muscles. It is the most common as
well as the most severe form of the four types.
o Local tetanus manifests with muscle spasms at or near the wound that has been
infected with the bacteria.
o Cephalic tetanus primarily affects one or several muscles in the face rapidly (in
one to two days) after a head injury or ear infection Trismus ("lockjaw") may
occur. The disease can easily progress to generalized tetanus.
o Neonatal tetanus is similar to generalized tetanus except that it affects a baby
that is less than 1 month old (called a neonate). This condition is rare in
developed countries.
6.5.2 Tetanus Causes
Clostridium tetani is the type of bacteria responsible for the disease. The bacteria are
found in two forms: as a spore (dormant) or as a vegetative cell (active) that can
multiply.
The spores are in soil, dust, and animal waste and can survive there for many years.
These spores are resistant to extremes of temperature.
Contamination of a wound with tetanus spores is rather common. Tetanus, however,
can only occur when the spores germinate and become active bacterial cells.
The active bacterial cells release two exotoxins, tetanolysin and tetanospasmin. The
function of tetanolysin is unclear, but tetanospasmin is responsible for the disease.
49
The disease typically follows an acute injury that results in a break in the skin. Most
cases result from a puncture wound, laceration (cut), or an abrasion (scrape).
Other tetanus-prone injuries include the following:
o frostbite,
o surgery,
o crush wound,
o abscesses,
o childbirth, and
o IV drug users (site of needle injection).
Wounds with devitalized (dead) tissue (for example, burns or crush injuries) or foreign
bodies (debris in them) are most at risk of developing tetanus.
Tetanus may develop in people who are not immunized against it or in people who
have failed to maintain adequate immunity with active booster doses of vaccine.
6.5.3 Tetanus Symptoms
The hallmark feature of tetanus is muscle rigidity and spasms.
In generalized tetanus, the initial complaints may include any of the following:
o
Irritability, muscle cramps, sore muscles, weakness, or difficulty swallowing
are commonly seen.
o Facial muscles are often affected first. Trismus or lockjaw is most common.
This condition results from spasms of the jaw muscles that are responsible for
chewing. A sardonic smile -- medically termed risus sardonicus -- is a
characteristic feature that results from facial muscle spasms.
o Muscle spasms are progressive and may include a characteristic arching of the
back known as opisthotonus. Muscle spasms may be intense enough to cause
bones to break and joints to dislocate.
o Severe cases can involve spasms of the vocal cords or muscles involved in
breathing. If this happens, death is likely, unless medical help (mechanical
ventilation with a respirator) is readily available.
In cephalic tetanus, in addition to lockjaw, weakness of at least one other facial muscle
occurs. In two-thirds of these cases, generalized tetanus will develop.
In localized tetanus, muscle spasms occur at or near the site of the injury. This
condition can progress to generalized tetanus.
Neonatal tetanus is identical to generalized tetanus except that it affects the newborn
infant. Neonates may be irritable and have poor sucking ability or difficulty
swallowing.
6.5.4 Diagnosis
The diagnosis of generalized tetanus is usually made by observing the clinical presentation
and a combination of the following:
history of a recent injury resulting in skin breakage (but this is not universal; only 70%
of cases have an identified injury);
incomplete tetanus immunizations;
progressive muscle spasms (starting in the facial region, especially lockjaw and
progressing outward from the face to include all muscles of the body);
50
fever;
changes in blood pressure (especially high blood pressure);
irregular heartbeat;
in localized tetanus, pain, cramps, or muscle spasms occur at or near a recent skin
injury;
neonates show signs of being generally irritable, muscle spasms, and poor ability to
take in liquids (poor sucking response), usually seen in neonates about 7-10 days old;
and
Laboratory tests: Though rarely used, some reference labs can determine if the patient
has serum antitoxin levels that are protective, and thus a positive test suggests that the
diagnosis of tetanus is unlikely.
6.5.5 Tetanus Treatment
6.5.5.1 Self-Care at Home
Any wound that results in a break in the skin should be cleaned with soap and running
water.
All open wounds are at risk to develop tetanus. Wounds from objects outdoors or
crush injuries are at higher risk.
Apply a clean and dry cloth to stop or minimize bleeding.
Apply direct pressure to the site of bleeding to help minimize blood loss.
6.5.5.2 Medical Treatment
antibiotics (for example, metronidazole) to kill the bacteria, tetanus booster shot, if
necessary, and occasionally, antitoxin to neutralize the toxin
wound cleansing to remove any obvious bacteria collections (abscesses) or foreign
bodies
supportive measures
pain medicine as needed
sedatives such as diazepam (Valium) to control muscle spasms
ventilator support to help with breathing in the event of spasms of the vocal cords or
the respiratory muscles
IV rehydration because, as muscles spasm constantly, increased metabolic demands
are placed on the body
6.5.6 Prevention
The majority of all adult types of tetanus cases can be prevented by active
immunization; neonatal cases are prevented by good hygiene and careful, sterile
technique used to sever the umbilical cord and later (at 2 months old), beginning
active immunizations.
For pediatric populations, DTaP (diphtheria, tetanus and acellular pertussis
combination vaccine) is used. For non-immunized adults and booster shots, Tdap
(tetanus and reduced amounts of diphtheria and acellular pertussis combination
vaccine) is recommended. Tdap was recommended (by the CDC in 2005) over the
older Td combination vaccine, as cases of pertussis (whooping cough) had been
increasing in the last decade.
51
All partially immunized as well as unimmunized adults should receive a tetanus
vaccination (see below).
The initial series for non-immunized adults involves three doses of Tdap:
o
o
o
The first and second doses are given four to eight weeks apart.
The third dose is given six months after the second.
Booster doses are required every 10 years after that.
In children, the immunization schedule calls for five doses of DTaP.
o
o
o
One dose is given at 2, 4, 6, and 15-18 months of age.
This DTaP series is completed with a final dose when the child is between 4-6
years of age.
Additional boosters with Tdap are given every 10 years after the final DTaP
dose. Children that miss doses of DTaP can be given Tdap doses, but the
choice for dose schedule should be determined by the patients' doctor.
People who are not completely immunized and have a tetanus-prone wound should receive a
tetanus booster in addition to tetanus antibodies (human tetanus immune globulin or TIG).
The tetanus antibodies (TIG) will provide short-term protection against the disease. For
patients sensitive to the combined vaccines (DTaP or Tdap), other vaccines against tetanus are
available (for example, Td), but the patients' doctor should determine the dosage schedule.
Vaccine shots are somewhat painful (pain likely due to multiple factors such as
inserting foreign material into a muscle, spreading out muscle fibers to make room for
vaccine volume, the body's immune response, and others), but that pain should never
prevent people from getting either immunized or booster shots. In most cases, the pain
does not last long.
6.5.7 Prognosis
Overall, about 25%-50% of people with generalized tetanus will die.
The disease is more serious when the symptoms come on quickly.
Older people and very young children tend to have more severe cases.
Intensive medical care improves the prognosis in severe cases.
Death is usually due to respiratory failure or disturbance of heart rhythm.
Data on worldwide neonatal deaths is not complete due to poor data collection in
many countries; however, several investigators suggest mortality rates range from
about 60%-80%.
6.5.8 Vaccine (Shot) Complications (Side Effects)
Problems with DTaP and Tdap range from mild to severe; the good news is that severe
problems (seizures, coma, brain damage, nerve problems, or severe allergic reactions)
occur in less than one in 1 million vaccinations. Many investigators think the severe
complications are so rare that it is difficult to prove they are actually related to the
vaccine administration. Consequently, the vast majority of physicians continue to
advocate the use of the vaccines.
The most frequent mild side effects of DTaP are pain, fever, fussiness in children, and
redness or swelling at the injection site. About one in four children may show some or
52
all of these effects, and they may be more prevalent after the fourth or fifth dose.
Other mild problems (feeling tired, decreased appetite, vomiting, fussiness) may occur
one to three days after the shot. Fussiness occurs most frequently (one in three
children), followed by tiredness and decreased appetite (one in 10), while vomiting is
infrequent (about one in 50). Moderate or uncommon effects of DTaP are a seizure or
high fever (105 F or higher); these occur in about one of 14,000 children vaccinated.
The most frequent mild side effects of Tdap are pain, redness, headache, chills, nausea
with occasional vomiting or diarrhea, swollen lymph glands, joint pain, and fever of
about 100.4 F. Mild side effects occur in about two in three to three in four
adolescents and adults while mild fever (100.4 F) may occur in one of 25 adolescents
and one of 100 adults. Moderate side effects of Tdap are pain, redness, swelling,
nausea, vomiting, diarrhea, and fever of 102 F or higher. Redness, swelling, and pain
occur slightly more frequently in adolescents (about one in 16 to 20) than in adults
(about one in 25 to 100). A similar frequency is seen with fever and gastrointestinal
side effects (about one to three per 100 adolescents) compared with fever in one in 250
adults and gastrointestinal side effects in one in 100 adults.
Most mild side effects of DTaP and Tdap usually require no treatment and are gone
within 24 hours; moderate side effects may be treated symptomatically, but a child
with a high fever or seizure should be evaluated and possibly treated by a physician.
Do not use aspirin to treat children's pain or fever.
6.6 Summary
Dysentery is an acute or chronic disease of the large intestine of humans, characterized
by frequent passage of small, watery stools, often containing blood and mucus,
accompanied by severe abdominal cramps. Ulceration of the walls of the intestine may
occur. Although many severe cases of diarrhea have been called dysentery, the word
properly refers to a disease caused by either a specific amoeba, Entamoeba histolytica,
or a bacillus that infects the colon.
Tetanus is an infectious disease caused by contamination of wounds from bacteria that
live in the soil. The causative bacterium Clostridium tetani is a hardy organism
capable of living many years in the soil in a form called a spore. The bacterium was
first isolated in 1899 by S. Kitasato while he was working with R. Koch in Germany.
Kitasato also found the toxin responsible for tetanus and developed the first protective
vaccine against the disease. Tetanus occurs when a wound becomes contaminated with
bacteria spores. Infection follows when spores become activated and develop into
gram-positive bacteria that multiply and produce a very powerful toxin (poison) that
affects the muscles. Tetanus spores are found throughout the environment, usually in
soil, dust, and animal waste. The usual locations for the bacteria to enter your body are
puncture wounds, such as those caused by rusty nails, splinters or insect bite. Burns,
any break in the skin, and IV drug access sites are also potential entryways for the
bacteria Tetanus is acquired through contact with environment; it is not transmitted
from person to person. Tetanus results in severe, uncontrollable muscle spasms. The
jaw is "locked" by muscle spasms, causing the disease to sometimes be called
"lockjaw." In severe cases, the muscles used to breathe can spasm, causing a lack of
oxygen to the brain and other organs that may possibly lead to death.
53
6.7 Self-Test Questions
i) Describe what amoebic and bacillary dysentery are.
ii) Explain the causes,transmission, symptoms and control of Amoeba and
Bacillary Dysentry
iii) Explain how tetanus is recognized.
iv) Explain how tetanus is transmitted.
v) Describe the prevention and control of tetanus.
54
LECTURE SEVEN
TROPICAL DISEASES: LEPROSY AND YAW
7.1 Introduction
Welcome to the seventh lecture in our course. In this lecture we will cover tropical diseases –
leprosy and yaw. The broader objective of this lecture is to enable you to describe tropical
diseases – leprosy and yaw.
7.2 Objectives
By end of this lecture you should be able to:
i.
ii.
Describe what leprosy and yaw are.
Explain causes, signs & symptoms, complications, diagnosis, treatment
prevention and control of leprosy and yaw.
7.3 LEPROSY (HANSEN'S DISEASE)
The following questions are important as you read through
What is leprosy?
What is the history of leprosy (Hansen's disease)?
What causes leprosy?
What are the symptoms and signs of leprosy?
Are there different forms (classifications) of leprosy?
How is leprosy transmitted?
How is leprosy diagnosed?
How is leprosy treated?
How is leprosy prevented?
7.3.1 What leprosy is
Leprosy is a disease caused by the bacteria Mycobacterium leprae that causes damage to the
skin and the peripheral nervous system. The disease develops slowly (from six months to 40
years!) and results in skin lesions and deformities, most often affecting the cooler places on
the body (for example, eyes, nose, earlobes, hands, feet, and testicles). The skin lesions and
deformities can be very disfiguring and are the reason that infected individuals were
considered outcasts in many cultures. Although human-to-human transmission is the primary
source of infection, three other species can carry and (rarely) transfer M. leprae to humans;
chimpanzees, mangabey monkeys, and nine-banded armadillos. The disease is termed a
chronic granulomatous disease because it produces inflammatory nodules (granulomas) in the
skin and nerves over time.
55
7.3.2 Causes of leprosy
Leprosy is caused by Mycobacterium leprae, a rod-shaped bacillus that is an obligate
intracellular (only grows inside of certain human and animal cells) bacterium. M. leprae is
termed an "acid fast" bacterium because of its chemical characteristics. When special stains
are used for microscopic analysis, it stains red on a blue background due to mycolic acid
content in its cell walls. The Ziel-Nielsen stain is an example of the special staining
techniques used to view the acid-fast organisms under the microscope.
Currently, the organisms cannot be cultured on artificial media. The bacteria take an
extremely long time to reproduce inside of cells (about 12-14 days as compared to minutes to
hours for most bacteria). The bacteria grow best at 80.9 F-86 F, so cooler areas of the body
tend to develop the infection. The bacteria grow very well in the body's macrophages and
Schwann cells (cells that cover and protect nerve axons). M. leprae is genetically related to M.
tuberculosis (the type of bacteria that cause tuberculosis) and other mycobacteria that infect
humans. As with malaria, patients with leprosy produce anti-endothelial antibodies
(antibodies against the lining tissues of blood vessels), but the role of these antibodies in these
diseases is still under investigation.
7.3.3 Symptoms and signs of leprosy
Unfortunately, the early signs and symptoms of leprosy are very subtle and occur slowly
(usually over years).
Numbness and loss of temperature sensation (cannot sense very hot or cold temperatures) are
some of the first symptoms that patients experience.
As the disease progresses, the sensation of touch, then pain and eventually deep pressure are
decreased or lost.
Signs that occur, such as relatively painless ulcers, skin lesions of hypopigmented macules
(flat, pale areas of skin), and eye damage (dryness, reduced blinking) are experienced before
the large ulcerations, loss of digits, and facial disfigurement develop.
This long-time developing sequence of events begins and continues on the cooler areas of the
body (for example, hands, feet, face, and knees).
7.3.4 Different forms (classifications) of leprosy
There are multiple forms of leprosy described in the literature. The forms of leprosy are based
on the person's immune response to M. leprae. A good immune response can produce the socalled tuberculoid form of the disease, with limited skin lesions and some asymmetric nerve
involvement. A poor immune response can result in the lepromatous form, characterized by
extensive skin and symmetric nerve involvement. Some patients may have aspects of both
forms. Currently, two classification systems exist in the medical literature: the WHO system
and the Ridley-Jopling system. The Ridley-Jopling system is composed of six forms or
classifications, listed below according to increasing severity of symptoms:
Indeterminate leprosy: a few hypopigmented macules; can heal spontaneously, persists
or advances to other forms
56
Tuberculoid leprosy: a few hypopigmented macules, some are large and some become
anesthetic (lose pain sensation); some neural involvement in which nerves become
enlarged; spontaneous resolution in a few years, persists or advances to other forms
Borderline tuberculoid leprosy: lesions like tuberculoid leprosy but smaller and more
numerous with less nerve enlargement; this form may persist, revert to tuberculoid
leprosy, or advance to other forms
Mid-borderline leprosy: many reddish plaques that are asymmetrically distributed,
moderately anesthetic, with regional adenopathy (swollen lymph nodes); the form may
persist, regress to another form, or progress
Borderline lepromatous leprosy: many skin lesions with macules (flat lesions) papules
(raised bumps), plaques, and nodules, sometimes with or without anesthesia; the form
may persist, regress or progress to lepromatous leprosy
Lepromatous leprosy: Early lesions are pale macules (flat areas) that are diffuse and
symmetric; later many M. leprae organisms can be found in them. Alopecia (hair loss)
occurs; often patients have no eyebrows or eyelashes; as the disease progresses, nerve
involvement leads to anesthetic areas and limb weakness; progression leads to aseptic
necrosis (tissue death from lack of blood to area), lepromas (skin nodules), and
disfigurement of many areas including the face; the lepromatous form does not regress
to the other less severe forms.
The Ridley-Jopling classification is used globally in evaluating patients in clinical studies.
However, the WHO classification system is more widely used; it has only two forms or
classifications of leprosy. The 2009 WHO classifications are simply based on the number of
skin lesions as follows:
Paucibacillary leprosy: skin lesions with no bacilli (M. leprae) seen in a skin smear
Multibacillary leprosy: skin lesions with bacilli (M. leprae) seen in a skin smear
However, the WHO further modifies these two classifications with clinical criteria because
"…of the non-availability or non-dependability of the skin-smear services. The clinical
system of classification for the purpose of treatment includes the use of number of skin
lesions and nerves involved as the basis for grouping leprosy patients into multibacillary
(MB) and paucibacillary (PB) leprosy." Investigators state that up to about four to five skin
lesions constitutes paucibacillary leprosy, while about five or more constitutes multibacillary
leprosy.
Mulitdrug therapy (MDT) with three antibiotics (dapsone, rifampicin, and clofazimine) is
used for multibacillary leprosy, while a modified MDT with two antibiotics (dapsone and
rifampicin) is recommended for paucibacillary leprosy. Paucibacillary leprosy usually
includes indeterminate, tuberculoid, and borderline tuberculoid leprosy from the RidleyJopling classification, while multibacillary leprosy usually includes the double (mid-)
borderline, borderline lepromatous, and lepromatous leprosy.
7.3.5 Transmmission of leprosy
Researchers suggest that M. leprae are spread person to person by nasal secretions or droplets.
They speculate that infected droplets reach other peoples' nasal passages and begin the
infection there. Some investigators suggest the infected droplets can infect others by entering
breaks in the skin. M. leprae apparently cannot infect intact skin. Rarely, humans get leprosy
57
from the few animal species mentioned above. Routes of transmission are still being
researched for leprosy.
7.3.6 Leprosy diagnosis
The majority of cases of leprosy are diagnosed by clinical findings, especially since most
current cases are diagnosed in areas that have limited or no laboratory equipment available.
Hypopigmented patches of skin or reddish skin patches with loss of sensation, thickened
peripheral nerves, or both clinical findings together often comprise the clinical diagnosis. Skin
smears or biopsy material that show acid-fast bacilli with the Ziel-Nelson stain or the Fite
stain (biopsy) can diagnose multibacillary leprosy, or if bacteria are absent, diagnose
paucibacillary leprosy.
Other tests can be done, but most of these are done by specialized labs and may help a
clinician to place the patient in the more detailed Ridley-Jopling classification and are not
routinely done (lepromin test, phenolic glycolipid-1 test, PCR, lymphocyte migration
inhibition test or LMIT). Other tests such as CBC test, liver function tests, creatinine test, or a
nerve biopsy may be done to help determine if other organ systems have been affected.
7.3.7 Treatment leprosy
The majority of cases (mainly clinically diagnosed) are treated with antibiotics. The
recommended antibiotics, their dosages and length of time of administration are based on the
form or classification of the disease and whether or not the patient is supervised by a medical
professional.
In general, paucibacillary leprosy is treated with two antibiotics, dapsone and rifampicin,
while multibacillary leprosy is treated with the same two plus a third antibiotic, clofazimine.
Usually, the antibiotics are given for at least six to 12 months or more.
Each patient, depending on the above criteria, has a schedule for their individual treatment, so
treatment schedules should be planned by a clinician knowledgeable about that patient's initial
diagnostic classification.
Antibiotics can treat paucibacillary leprosy with little or no residual effects on the patient.
Multibacillary leprosy can be kept from advancing, and living M. leprae can be essentially
eliminated from the person by antibiotics, but the damage done before antibiotics are
administered is usually not reversible. Recently, the WHO suggested that single-dose
treatment of patients with only one skin lesion with rifampicin, minocycline (Minocin), or
ofloxacin (Floxin) is effective. Studies of other antibiotics are ongoing.
The role for surgery in the treatment of leprosy occurs after medical treatment (antibiotics)
has been completed with negative skin smears (no detectable acid-fast bacilli) and is often
only needed in advanced cases. Surgery is individualized for each patient with the goal to
attempt cosmetic improvements and, if possible, to restore limb function and some neural
functions that were lost to the disease.
58
7.3.8 Prevention leprosy
Prevention of contact with droplets from nasal and other secretions from patients with
untreated M. leprae infection currently is a way to avoid the disease. Treatment of patients
with appropriate antibiotics stops the person from spreading the disease. People that live with
individuals that have untreated leprosy are about eight times as likely to develop the disease,
because investigators speculate that family members have close proximity to infectious
droplets. Leprosy is not hereditary.
Many people get exposed to leprosy throughout the world, but the disease in not highly
contagious; researchers suggest that over 95% of exposures result in no disease. In the U.S.,
there are about 200-300 new cases diagnosed per year, with most coming from exposures
during foreign travel. The majority of worldwide cases are found in the tropics or subtropics
(for example, Brazil, India, and Indonesia). The WHO reports about 500,000 to 700,000 new
cases per year worldwide, with curing of about 14 million cases since 1985.
There is no vaccine available to prevent leprosy. Animals (chimpanzees, mangabey monkeys,
and nine-banded armadillos) rarely transfer M. leprae to humans; nonetheless, handling such
animals in the wild is not advised.
7.3.9 Leprosy in Kenya
In Kenya, Leprosy is still found in a few districts in the following provinces:
a)
b)
c)
d)
e)
Coast
Nyanza
Western
Eastern
Nairobi
Kenya is in the post elimination phase since prevalence is less than 1/10,000 population
corresponding to the WHO definition of elimination. However, leprosy has not been
eradicated in Kenya.
Its likely that many leprosy cases are not detected and that the true incidence is much higher
than current, so the true prevalence and incidence is not known. Initiatives have thus been put
in place to carry out this exercise, some of the initiativies already developed include: capacity
building on health workers, creating awareness by developing IEC materials and provinding
footwear to leprosy patients.Some initiatives have been rolled out e.g. Training of DTLCs in
all the districts where leprosy cases are registered.
Since the introduction of Multiple Drug Therapy in 1984, the registered prevalence decreased
from 6,558 cases in 1986 to 148 cases by the end of 2009. The sudden drop is because of
change in definition of a leprosy case which led to many patients being released from
treatment. The number of new leprosy cases detected decreased from 630 in 1986 to 204 in
2009.
59
7.3.10 Conclusion
Leprosy is a slowly developing, progressive disease that damages the skin and nervous
system.
Leprosy is caused by an infection with Mycobacterium leprae bacteria.
Early symptoms begin in cooler areas of the body and include loss of sensation.
Signs of leprosy are painless ulcers, skin lesions of hypopigmented macules (flat, pale
areas of skin) and eye damage (dryness, reduced blinking). Later, large ulcerations,
loss of digits, skin nodules, and facial disfigurement may develop.
The infection is spread person to person by nasal secretions or droplets.
Antibiotics are used in the treatment of leprosy.
7.4 YAWS
Get answers to the following questions as we discuss through this section.
What is yaws? What are symptoms of yaws?
What causes yaws?
How does yaws begin and spread?
What are developmental stages in the course of yaws?
How is yaws diagnosed?
How is yaws treated?
Why is yaws a serious problem?
Why this disease is called yaws?
7.4.1 What yaws is
Yaws is a common chronic infectious disease that occurs mainly in warm humid
regions such as the tropical areas of Africa, Asia, South and Central Americas, plus
the Pacific Islands.
The disease has many names (for example, pian, parangi, paru, frambesia tropica).
Yaws usually features lesions that appear as bumps on the skin of the face, hands, feet,
and genital area.
The disease most often starts as a single lesion that becomes slightly elevated,
develops a crust that is shed, leaving a base that resembles the texture of a raspberry or
strawberry.
This primary lesion is termed the mother yaw (also termed buba, buba madre, or
primary frambesioma).
Secondary lesions, termed daughter yaws, develop in about six to 16 weeks after the
primary lesion.
Almost all cases of yaws begin in children under 15 years of age, with the peak
incidence in 6-10-year-old children. The incidence is about the same in males and
females.
60
7.4.2 Causes of yaws
Yaws is caused by a particular bacterium called a spirochete (a spiral-shaped type of
bacteria).
The bacterium is scientifically referred to as Treponema pertenue. This organism is
considered by some investigators to be a subspecies of T. pallidum, the organism that
causes syphilis (a systemic sexually-transmitted disease).
Other investigators consider it to be a closely related but separate species of
Treponema. T. carateum, the cause of pinta (a skin infection with bluish-black spots),
is also closely related to T. pertenue.
The history of yaws is unclear; the first possible mention of the disease is considered
to be in the Old Testament. D. Bruce and D. Nabarro discovered the spirochete
causing yaws (T. pertenue) in 1905.
7.4.3 Transmission
Yaws begins when T. pertenue penetrates the skin at a site where skin was scraped,
cut, or otherwise compromised. In most cases, T. pertenue is transmitted from person
to person. At the entrance site, a painless bump lesion, or bump, arises within two to
eight weeks and grows.
The initial lesion is referred to as the mother yaw. The lymph nodes in the area of the
mother yaw are often swollen (regional lymphadenopathy). When the mother yaw
heals, a light-colored scar remains.
7.4.4 Developmental stages in the course of yaws
Yaws has four stages:
i.
ii.
iii.
iv.
Primary,
Secondary,
Latent, and
Tertiary.
The primary stage is the appearance of the mother yaw.
Patients with yaws develop recurring ("secondary") lesions and more swollen lymph
nodes. This represents the secondary stage. These secondary lesions may be painless
like the mother yaw or they may be filled with pus, burst, and ulcerate. The affected
child often experiences malaise (feels poorly) and anorexia (loss of appetite).
The latent stage occurs when the disease symptoms abate, although an occasional
lesion may occur.
In the tertiary stage, yaws can destroy areas of the skin, bones, and joints and deform
them. The palms of the hands and soles of the feet tend to become thickened and
painful (crab yaws).
7.4.5 Diagnosis of yaws
Yaws is suspected in any child who has the characteristic clinical features and lives in
an area where the disease is common.
61
With increasing travel, a child once in the tropics may carry the disease to a more
temperate area of the world.
Laboratory confirmation of the diagnosis is by blood serum tests (for example, RPR or
rapid plasma reagent test, VDRL test or venereal disease research laboratory test,
TPHA or Treponema pallidum hemagglutination test, FTA-ABS or fluorescent
treponema antibody absorption), but most frequently the diagnosis is made on clinical
findings.
The reason that T. pallidum serum tests are used is that the spirochetes are so closely
related, they have similar antigens on their surfaces so that T. pallidum and T.
pertenue are cross-reactive (detected by the same serological tests).
Special (dark-field) examination under the microscope in which technicians can
actually see the spirochete bacterium is also used to help diagnose yaws.
The lesions (both the mother yaw and the secondary lesions) usually have many T.
pertenue organisms that can be visualized with dark-field examination of lesion
scrapings.
On a typical Gram stain (a procedure for identifying bacteria when viewed
microscopically), the organisms are considered to be Gram-negative but stain so
poorly and are so small and thin, the Gram stain often does not reveal the organisms;
hence the use of the dark-field examination.
Other tests that detect spirochetes such as a silver stain or electron microscopy are
used mainly by research scientists.
7.4.6 Treatment of yaws
Treatment of yaws is simple and highly effective.
Penicillin G benzathine given IM (intramuscularly) can cure the disease in the
primary, secondary, and usually in the latent phase. Penicillin V can be given orally
for about seven to 10 days, but this route is less reliable than direct injection.
Anyone allergic to penicillin can be treated with another antibiotic, usually
erythromycin, doxycycline, or tetracycline.
Tertiary yaws, which occurs in about 10% of untreated patients five to 10 years after
initially getting the disease, is not contagious.
The tertiary yaws patient is treated for the symptoms of the chronic conditions (altered
or destroyed areas in bones, joints, cartilage, and soft tissues) that develop as a result
of the infection. There is no vaccine for yaws.
7.4.7 Yaws a serious public health problem
Yaws is a major public-health threat in the tropics. Tropical regions in Central and
South America, Africa, Asia, and Oceania are all at continuing risk for yaws.
A high percentage of children in such areas can be infected.
Transmission of the disease is facilitated by overcrowding and poor hygiene, and yaws
tends to be more prevalent in poor areas.
In addition to making young children sick, approximately 10% of untreated children
develop into young adults with deformities that are severely debilitating in the tertiaryyaws phase.
For example, some patients develop destructive ulcerations of the nasopharynx, palate
and nose (termed gangosa), painful skeletal deformities, especially in the legs (termed
saber shins), and other soft-tissue changes (gummas, inflammatory cell infiltration).
62
Yaws can be completely eradicated from an area by giving penicillin or other
appropriate antibiotics to everyone in the population.
This may, unfortunately, cost more than a poor country can afford.
From 1950-1970, a worldwide effort to eradicate yaws was begun and made progress
in reducing the approximately 50 million worldwide cases; after its end, yaws has seen
a resurgence.
In the 1990s, attempts to eliminate yaws started again, with limited success as the
effort is not worldwide or coordinated but done by individual countries.
The WHO (World Health Organization) in 2007 reported about 2.5 million cases
worldwide but freely admits their data is faulty, as most countries do not calculate the
prevalence of yaws. W.H.O. estimates that about 460,000 new cases of yaws occur
each year.
7.4.8 Conclusion
Yaws is a common disease of children in the tropics.
Yaws is a chronic, relapsing infectious illness.
Yaws first affects the skin and later possibly the bones and joints as well.
Yaws is caused by a bacterium, the spirochete Treponema pertenue.
Transmission is by skin-to-skin contact. The spirochete cannot penetrate
normal skin but can enter through a scrape or cut in the skin.
Yaws is promoted by overcrowding and poor hygiene.
Yaws (except for tertiary yaws) can be cured by a single shot of
penicillin.
7.5 Summary
Also Leprosy is a slowly developing, progressive disease that damages the skin and
nervous system. Leprosy is caused by an infection with Mycobacterium leprae
bacteria. Early symptoms begin in cooler areas of the body and include loss of
sensation. Signs of leprosy are painless ulcers, skin lesions of hypopigmented macules
(flat, pale areas of skin) and eye damage (dryness, reduced blinking). Later, large
ulcerations, loss of digits, skin nodules, and facial disfigurement may develop. The
infection is spread person to person by nasal secretions or droplets. Antibiotics are
used in the treatment of leprosy.
Yaws is an infectious disease that mainly occurs in the tropical areas of South and
Central America, Asia, Africa, and the Pacific Islands. The disease is caused by a
bacterium called Treponema pertenue, which causes lesions that look like bumps on
the skin of the feet, hands, face, and genital area. Yaws is treated with penicillin or
another antibiotic.
63
7.6 Self-Test Questions
i.
ii.
iii.
iv.
v.
vi.
vii.
What is leprosy?
What causes leprosy?
How is leprosy transmitted?
How is leprosy prevented?
Explain how yaw is recognized
Explain howYaw is transmitted.
Describe the prevention and control of yaw
64
LECTURE EIGHT
HELMINTHIASIS
8.1 Introduction
Welcome to the eighth lecture in our course. In this lecture we will cover Helminthiasis. The
broader objective of this lecture is to enable you to describe Helminthiasis
8.2 Objectives
By end of this lecture you should be able to:
i)
ii)
iii)
iv)
v)
Describe what helminthiasis is.
Explain the causes of helminthiasis.
Explain complications resulting from helminthiasis.
Describe the prevation and control of helminthiasis.
Describe various infections resulting from helminthiasis
8.3 HELMINTHIASIS
Helminthiasis is infestation with one or more intestinal parasitic worms (roundworms
(Ascaris lumbricoides), whipworms (Trichuris trichiura), or hookworms (Necator
americanus and Ancylostoma duodenale).
Infected people excrete helminth eggs in their faeces, which then contaminate the soil
in areas with inadequate sanitation. Other people can then be infected by ingesting
eggs or larvae in contaminated food, or through penetration of the skin by infective
larvae in the soil (hookworms).
Infestation can cause morbidity, and sometimes death, by compromising nutritional
status, affecting cognitive processes, inducing tissue reactions, such as granuloma, and
provoking intestinal obstruction or rectal prolapse.
Control of helminthiasis is based on drug treatment, improved sanitation and health
education.
Helminthiasis is a disease in which a part of the body is infested with worms such as
pinworm, roundworm or tapeworm. Typically, the worms reside in the gastrointestinal
tract but may also burrow into the liver and other organs.
Several types of soil-transmitted helminthiases are considered neglected diseases.
8.4 Types of Infection
Schistosomiasis
Onchocerciasis
Filariasis
Ascariasis
Trichuriasis
Hookworm
65
8.5 SCHISTOSOMIASIS
8.5.1 What is schistosomiasis?
Schistosomiasis is a tropical disease that can cause serious, long-term illness. Schistosomiasis
is also called bilharzia. Is a parasitic disease that leads to chronic ill health. It is the major
health risk in the rural areas of many developing countries. Schistosomiasis has been
recognized since the time of the Egyptian pharaohs. The worms responsible for the disease
were eventually discovered in 1851 by Theodor Bilharz, a young German pathologist, from
whom the disease took its original name, Bilharziasis. The disease is indicated either by the
presence of blood in the urine or, in the case of intestinal schistosomiasis, by initially atypical
symptoms which can lead to serious complications involving the liver and spleen.
8.5.2 Infectious agent that causes schistosomiasis
The main forms of human schistosomiasis are caused by five species of the flatworm, or
blood flukes, known as schistosomes:
Schistosoma mansoni causes intestinal schistosomiasis and is prevalent in 52
countries and territories of Africa, Caribbean, the Eastern Mediterranean and South
America
Schistosoma japonicum/Schistosoma mekongi cause intestinal schistosomiasis and
are prevalent in 7 African countries and the Pacific region
Schistosoma intercalatum is found in ten African countries
Schistosoma haematobium causes urinary schistosomiasis and affects 54 countries in
Africa and the Eastern Mediterranean.
People are infected by contact with water used in normal daily activities such as personal or
domestic hygiene and swimming, or by professional activities such as fishing, rice cultivation,
and irrigation. Due to lack of information or insufficient attention to hygiene, infected
individuals may contaminate their water supply with faeces or urine. The eggs of the
schistosomes (above) in the excreta of an infected person open on contact with water and
release a parasite, the miracidium. To survive, this motile form must find a fresh water snail:
Once it has found its snail host, the miracidium divides, producing thousands of new parasites
(cercariae). The cercariae are then excreted by the snail into the surrounding water. They can
penetrate an individual's skin within a few seconds, continuing their biological cycle once
they have made their way to the victim's blood vessels. Within 30 to 45 days, the parasite is
transformed into a long worm which is either male or female. The female lays from 200 to
2000 eggs per day over an average of 5 years, according to the species.
In the case of intestinal schistosomiasis, the worms reside in the blood vessels lining the
intestine. In urinary schistosomiasis, they live in the blood vessels of the bladder. Only about
a half of the eggs are excreted in the faeces (intestinal schistosomiasis), or in the urine
(urinary schistosomiasis). The rest stay in the body, damaging other vital organs. It is the eggs
and not the worm itself which cause damage to the intestines, the bladder and other organs.
Schistosomiasis is endemic in 74 tropical developing countries. Some 600 million people are
at risk of becoming infected. It is estimated that 200 million people are already infected.
Extreme poverty, the unawareness of the risks, the inadequacy or total lack of public health
66
facilities plus the unsanitary conditions in which millions of people lead their daily lives are
all factors contributing to the risk of infection.
Schistosomiasis mainly affects adults workers in rural areas, employed either in agriculture or
the freshwater fishing sector. In many areas, a high proportion of children between the ages of
10 and 14 are infected. Urinary schistosomiasis affects 66 million children throughout 54
countries. In some villages around Lake Volta in Ghana, over 90% of the children are infected
by the disease.
As with other tropical diseases, population movements and refugees in unstable regions
contribute to the transmission of schistosomiasis. Rapid urbanization has been accompanied
by new foci of transmission. The increase in "off-track" tourism has led to increasingly
serious infections with previously unencountered sequelae, including paralysis of the legs.
The large fresh water reservoirs associated with dams such as Akosombo Dam in Ghana, the
Kainji Dam in Nigeria and the Kariba Dam in Zimbabwe as well as smaller reservoirs in the
Sahel and irrigation systems throughout Africa are major transmission foci and thus endemic
areas for schistosomiasis.
Although the majority of people in endemic areas have only light infections or no symptoms,
the impact of schistosomiasis on economic conditions and the general health situation should
not be underestimated. In the north-east of Brazil, in Egypt and the Sudan, the work capacity
of rural workers has been estimated to be seriously undermined. The disease also substantially
affects children's growth and school performance. However, medical treatment is rapidly
followed by improvement.
There is an association between urinary schistosomiasis and a form of cancer of the bladder in
some regions. This link is mainly recorded among the active section of the population, most
of whom are farmers. In Egypt, schistosomiasis linked with cancer is the primary cause of
death among men aged between 20 and 44 years. In the industrialized countries, cancer of the
bladder without schistosomiasis is usually prevalent among workers aged around 65. In some
regions of Africa where Schistosoma haematobium is prevalent, the incidence of cancer of the
bladder linked to schistosomiasis is 32 times higher than the incidence of cancer of the
bladder in the USA.
8.5.3 Distribution of schistosomiasis.
Schistosomiasis is found in these parts of the world:
Africa: Southern Africa, sub-Saharan Africa, Lake Malawi, Nile River valley in Egypt
Latin America: Brazil, Suriname, Venezuela, Antigua, Dominican Republic,
Guadeloupe
Caribbean: Martinique, Montserrat, Puerto Rico, St. Lucia (low risk)
Middle East: Iran, Iraq, Saudi Arabia, Syrian Arab Republic, Yemen
Southern China
Southeast Asia: Philippines, Laos, Cambodia, Japan, central Indonesia, Mekong delta
67
8.5.4 Transmission of schistosomiasis
People get schistosomiasis by skin contact with contaminated fresh water in which certain
types of snails that carry schistosomes are living.
Infected people pass Schistosoma eggs in their urine and stool. The eggs get into fresh water
sources when infected people urinate or defecate in the water. The eggs hatch in the water and
seek out the snails they need to survive. Once in the snails, the parasites grow, reproduce, and
are released into the water, where they can live for about 48 hours.
The parasites can penetrate the skin of persons who are using the water for washing or
bathing, swimming, or work activities such as fishing, rice cultivation, or irrigation. Within
several weeks, worms grow inside the blood vessels of the body and produce eggs. Some of
these eggs travel to the bladder or intestines and are passed into the urine or stool.
Only about half of the eggs are passed in the urine or stool. The rest stay in the body where
they can scar and damage vital organs. The symptoms of the disease are caused by the body's
reaction to the worms' eggs, not by the worms themselves.
8.5.4.1 Life cycle
Schistosomes have a typical trematode vertebrate-invertebrate lifecycle, with humans being
the definitive host (Figure 8.1).
8.5.5 Signs and symptoms of schistosomiasis
Within days after becoming infected, some people have a rash or itchy skin. Fever, chills,
cough, and muscle aches can begin within 1-2 months of infection. Most people have no
symptoms at this early phase of infection.
Later symptoms are related to the number and location of parasite eggs in the body. Eggs
travel to the liver or pass into the intestine or bladder, causing symptoms related to these
organs. In rare cases, eggs can travel to the brain or spinal cord and cause seizures, paralysis,
or spinal cord inflammation.
8.5.6 Diagnosis of schistosomiasis
Schistosomiasis is diagnosed by testing the urine or stool for parasites. A blood test has been
developed and is available. For accurate results, a blood sample should be taken 6-8 weeks
after the last exposure to contaminated water.
8.5.7 Who is at risk for schistosomiasis?
Persons who live in or travel to areas where schistosomiasis occurs and who have skin contact
with fresh water (river, streams, lakes, canals) are at risk of getting schistosomiasis.
68
Figure 8.1: Schistosomiasis life cycle.
Source: CDC
8.5.8 Treatment of schistosomiasis
The disease is treatable, usually with the drug praziquantel taken for 1-2 days. Until the
1970's, treatment of schistosomiasis was nearly as dangerous as the disease itself. Modern
treatment is effective and without risk.
Three new drugs have revolutionized treatment:
Praziquantel - effective in the treatment of all forms of schistosomiasis, with virtually
no side effects
Oxamniquine - used exclusively to treat intestinal schistosomiasis in Africa and
South America
Metrifonate - effective for the treatment of urinary schistosomiasis
Modern diagnostic techniques are simple, easy to apply and cost very little. Although
reinfection may occur after treatment, the risk of serious disease developing in the body
organs has been greatly reduced, and it has been observed that there is a marked regression of
lesions in young children following treatment of the infection. In the majority of localities
69
where treatment is provided, the total number of cases is reduced within 18 to 24 months. In
other localities, according to the local situation, the prevalence has been substantially reduced,
and it is encouraging to note that no further intervention is required for intervals between 2
and 5 years.
To be effective, schistosomiasis control strategies should be adapted to the local
epidemiological situation and caution must be taken when destroying freshwater snails using
chemicals - particularly in terms of impact on the environment.
In 1983, the World Health Organization, in association with the health ministries of several
endemic countries (Botswana, Egypt, Madagascar, Mauritius and Zanzibar), launched a
massive programme to assess control methods. The findings of this programme are as
follows:
Single dose praziquantel is effective in reducing prevalence and in containing the
disease
Schistosomiasis treatment must be accompanied by health education.
Praziquantel can be safely administered by primary healthcare teams
It is almost always necessary to repeat the treatment, but the interval may be up to 5
years in some situations where transmission is low.
A simple and rapid test for blood in the urine using a paper strip soaked in a reactive agent is
more reliable than a microscopic examination, which can prove difficult in the field.
8.5.9 Complications resulting from schistosomiasis
For those who go without treatment for a long time, schistosomiasis can be hard to cure.
There can be lifelong damage to the liver, lungs, intestines, or bladder. For those who are
exposed only briefly, such as during travel, and who are not reinfected, complications are rare
even without treatment.
The presence of widespread schistosomiasis in a country is usually a sign of problems in
sanitary waste disposal and treatment. The long-term illnesses that result from the infection
can have serious consequences for a country's socioeconomic development.
8.5.10 Schistosomiasis as an emerging infectious disease
Schistosomiasis is an increasing problem around the world as countries build and develop
new agricultural and water resources and as more people are exposed to infection. Refugee
movement in Africa, the Eastern Mediterranean, and Asia, and construction of dams,
reservoirs, and irrigation systems are introducing schistosomiasis to new areas and increasing
the spread of infection. The growing popularity of wilderness tourism is exposing more
travelers.
8.5.11 Prevention and Control of Schistosomiasis
The priorities are:
health education
the supply of drinking water and the planning of adequate healthcare facilities
70
diagnosis and treatment
management of the environment
control of the intermediate hosts (freshwater snails)
Schistosomiasis control is far more effective when placed in the context of a general health
system. The integration process is slow, but this "horizontal" approach is now becoming an
integral part of health care at village level. Schistosomiasis prevention and control measures
should be implemented before dam construction work begins. Control approaches for each
form of schistosomiasis vary and must be adapted to the epidemiological situation, available
financial resources, and the particular local culture. This strategy has produced excellent
results; in some regions it has met the planned objectives within 2 years. It is nevertheless
essential to plan surveillance and maintenance over periods of 10 to 20 years.
Health education on schistosomiasis has greater importance than ever before. The
introduction into schools of diagnosis and treatment has made children and parents much
more aware of the problem connected with disease. Schoolteachers and local health workers
are effective in explaining the role played by people in the transmission of schistosomiasis.
Campaigns in the Egyptian mass media have proved particularly successful in increasing
awareness of the need for diagnosis and treatment.
The supply of safe drinking water is fundamental to schistosomiasis control. The beneficial
results of chemotherapy - normally quite spectacular - are even more marked in communities
with adequate water supplies. The high prevalence of schistosomiasis is clearly a reliable
criterion to select communities for installing a clean water supply.
Follow these precautions when in countries where schistosomiasis occurs:
Avoid swimming or wading in fresh water. Swimming in the ocean and in chlorinated
swimming pools is generally thought to be safe.
Drink safe water. Because there is no way to make sure that water coming directly
from canals, lakes, rivers, streams, or springs is safe, either boil water for 1 minute or
filter water before drinking it. Boiling water for at least 1 minute will kill any
parasites, bacteria, or viruses present. Iodine treatment alone will not guarantee that
water is safe and free of all parasites.
Heat bath water for 5 minutes at 150o F. Water held in a storage tank for at least 48
hours should be safe for showering.
Vigorous towel drying after an accidental, very brief water exposure might help to
prevent the Schistosoma parasite from penetrating the skin. However, do not rely on
vigorous towel drying to prevent schistosomiasis.
8.5.12 Conclusion
Schistosomiasis is a disease of the tropics that can lead to serious, long-term illness.
Schistosomiasis is caused by parasitic flatworms.
People get schistosomiasis by contact with fresh water containing flatworm eggs.
Schistosomiasis is treatable.
To prevent schistosomiasis, avoid swimming or wading in fresh water when visiting
areas where the disease is known to occur.
71
8.6 ONCHOCERCIASIS (RIVER BLINDNESS)
8.6.1 Infectious Agent
Onchocerciasis, also known as river blindness, is caused by the filarial nematode, Onchocerca
volvulus.
8.6.2 Mode of Transmission
Infection occurs through vector-borne transmission by the bite of female blackflies of the
genus Simulium that bite during the day and are found near rapidly flowing rivers and
streams.
8.6.3 Distribution
Onchocerciasis is endemic in more than 25 nations located in a broad band across the
central part of Africa. Small endemic foci are also present in the Arabian Peninsula
(Yemen) and in the Americas (Brazil, Colombia, Ecuador, Guatemala, southern
Mexico, and Venezuela).
An estimated 17 million people are infected worldwide.
8.6.4 Risk for Travelers
Short-term travelers to endemic areas are at low risk for this infection.
Travelers who visit endemic areas for extended periods of time (generally greater than
3 months) and live or work near blackfly habitats are at greater risk for infection.
Most infections seen in the United States occur in expatriate groups, such as
missionaries, field scientists, and Peace Corps volunteers.
8.6.5 Signs and Symptoms
Infection with O. volvulus can result in a highly pruritic, papular dermatitis;
subcutaneous nodules; lymphadenitis; and ocular lesions, which can progress to visual
loss and blindness.
Symptoms in travelers are primarily dermatologic and may occur months to years after
departure from endemic areas.
Immigrants from endemic areas may present with skin and/or ocular disease.
8.6.6 Diagnosis
Diagnosis is made by finding either the microfilariae in superficial skin shavings or
punch biopsy, adult worms in histologic sections of excised nodules, or characteristic
eye lesions.
Serologic testing is most useful for detecting infection in specific groups, such as
expatriates with a brief exposure history, when microfilariae are not identifiable.
Determination of serum antifilarial immunoglobulin (IgG) is available through the
Parasitic Diseases Laboratory at the National Institutes of Health (NIH) or through the
Division of Parasitic Diseases, CDC.
72
8.6.7 Treatment
Ivermectin (150–200 μg/kg orally, once or twice per year) is the drug of choice for
onchocerciasis. Repeated annual or semiannual doses may be required, because the
drug kills the microfilariae but not the adult worms, which can live for many years.
Antibiotic trials, with doxycycline (100 mg orally per day), directed against
Wolbachia, an endosymbiont of O. volvulus, have demonstrated a decrease in
onchocercal microfiladermia with 6 weeks of therapy.
Diethylcarbamazine (DEC) is contraindicated in onchocerciasis, as it has been
associated with severe and fatal post-treatment reactions.
Any subcutaneous nodules should be excised if their anatomic location allows it to be
done safely.
To ensure correct diagnosis and treatment, travelers should be advised to consult with
an infectious diseases or tropical medicine specialist.
8.6.8 Preventive Measures
No vaccine is available.
No drugs for preventing infection are available.
Protective measures include avoidance of blackfly habitats and the use of personal
protection measures against biting insects (see the Protection Against Mosquitoes,
Ticks, and Other Insects and Arthropods section in Chapter 2)..3 Lymphatic Filariasis
8.7 LYMPHATIC FILARIASIS
Filariasis is an infection with any of several round, thread-like parasitic worms. The most
common type of filariasis is infection with a parasitic worm that lives in the human lymph
system. This is called lymphatic filariasis.
8.7.1 Infectious agent that causes lymphatic filariasis
Filariasis is caused by three types of parasitic worms: Wuchereria bancrofti, Brugia malayi,
and Brugia timori.
8.7.2 Distribution of lymphatic filariasis
Lymphatic filariasis is a disease of the tropics. Wuchereria bancrofti, the most common
filariasis parasite, is found in Africa, India, Southeast Asia, the Pacific islands, the Caribbean,
and South America. The Brugia malayi and Brugia timori parasites are found in Southeast
Asia.
8.7.3 How lymphatic filariasis is spread
Filariasis is spread from infected persons to uninfected persons by mosquitoes. Adult worms
live in an infected person's lymph vessels. The females release large numbers of very small
worm larvae, which circulate in an infected person's bloodstream. When the person is bitten
by a mosquito, the mosquito can ingest the larvae. These develop in the mosquito and can
then be spread to other people via mosquito bites. After a bite, the larvae pass through the
73
skin, travel to the lymph vessels, and develop into adults, which live about 7 years. Then the
cycle begins again.
8.7.4 Signs and symptoms of lymphatic filariasis
Most of the signs and symptoms of filariasis are caused as a consequence of the adult worms
living in the lymph system. Tissue damage caused by the worms restricts the normal flow of
lymph fluid. This results in swelling, scarring, and infections. The legs and groin are most
often affected.
8.7.5 Incubation period
Symptoms can appear 5-18 months after a mosquito bite.
8.7.6 Diagnosis lymphatic filariasis
Filariasis larvae can sometimes be detected in blood.
8.7.7 People at risk for lymphatic filariasis
Those most at risk are people who live in or stay for a long time in tropical areas where the
disease is common. Short-term tourists rarely get filariasis. Getting an infection with
symptoms usually requires several mosquito bites over a long period of time.
8.7.8 Complications resulting from lymphatic filariasis
Lymphatic filariasis is rarely fatal, but it can cause recurring infections, fevers, severe
inflammation of the lymph system, and a lung condition called tropical pulmonary
eosinophilia (TPE). In about 5% of infected persons, a condition called elephantiasis causes
the legs to become grossly swollen. This can lead to severe disfigurement, decreased mobility,
and long-term disability. Testicular hydrocele is a disfiguring enlargement of the scrotum.
8.7.9 Treatment for lymphatic filariasis
Treatment consists of:
•
•
•
Medicine to kill circulating larvae and adult worms,
Soap and water and skin care to prevent secondary infections, and
Elevation, exercises, and, in some cases, pressure bandages to reduce swelling.
8.7.10 How common lymphatic filariasis is.
At least 120 million people in 73 countries worldwide are estimated to be infected with
filariasis parasites. The most widespread is Wuchereria bancrofti, which affects about 100
million people in Africa, India, Southeast Asia, the Pacific islands, South America, and the
Caribbean. The Brugia malayi and Brugia timori parasites affect about 12 million people in
Southeast Asia.
74
8.7.12 Lymphatic filariasis as an emerging infectious disease
Lymphatic filariasis is among the world's leading causes of permanent and long-term
disability. The number of infected persons is increasing worldwide, due in large part to
unchecked urbanization in many areas where the parasite is common.
8.7.13 Prevention of lymphatic filariasis
There is no vaccine for filariasis. Prevention centers on mass treatment with anti-filariasis
drugs to prevent ingestion of larvae by mosquitoes, public health action to control mosquitoes,
and individual action to avoid mosquito bites. To avoid being bitten by mosquitoes:
If possible, stay inside between dusk and dark. This is when mosquitoes are most
active in their search for food.
When outside, wear long pants and long-sleeved shirts.
Spray exposed skin with an insect repellent.
8.7.14 Conclusion
Lymphatic filariasis is a disease of the tropics. It is caused by infection with any of
several round, thread-like parasitic worms. The most common is infection with a
parasite that lives in the lymph system. This is called lymphatic filariasis.
The parasite is spread from person to person by infected mosquitoes.
Long-term exposure and repeated infections can cause severe damage to the lymph
system and serious, debilitating complications.
Prevention centers on controlling mosquito populations in communities and avoiding
mosquito bites.
8.8 ASCARIASIS
8.8.1 what Ascariasis is
Ascariasis is a human disease caused by the parasitic roundworm Ascaris
lumbricoides.
Approximately as many as one quarter of the world's people are infected
Ascariasis is particularly prevalent in tropical regions and in areas of poor hygiene.
Other species of the genus Ascaris are parasitic and can cause disease in domestic
animals.
8.8.2 Transmission
Infection occurs through ingestion of food contaminated with feces containing Ascaris
eggs. The larvae hatch, burrow through the intestine, reach the lungs, and finally
migrate up the respiratory tract.
From there they are then reswallowed and mature in the intestine, growing up to 30 cm
(12 in.) in length and anchoring themselves to the intestinal wall.
Infections are usually asymptomatic, especially if the number of worms is small. They
may however be accompanied by inflammation, fever, and diarrhea, and serious
problems may develop if the worms migrate to other parts of the body.
75
8.8.3 Distribution
Roughly 1.5 billion individuals are infected with this worm, primarily in Africa and
Asia.
Ascariasis is endemic in the United States including Gulf Coast; in Nigeria and in
Southeast Asia.
Deposition of ova (eggs) in sewage hints at the degree of ascariasis incidence.
Ascariasis can often be measured by examining food for ova. In one field study in
Marrakech, Morocco, where raw sewage is used to fertilize crop fields, Ascaris eggs
were detected at the rate of 0.18 eggs/kg in potatoes, 0.27 eggs/kg in turnip, 4.63
eggs/kg in mint, 0.7 eggs/kg in carrots, and 1.64 eggs/kg in radish[6]. A similar study
in the same area showed that 73% of children working on these farms were infected
with helminths, particularly Ascaris, probably as a result of exposure to the raw
sewage.
8.8.4 Life cycle
-
Adult worms (1)
-
live in the lumen of the small intestine. A female may produce approximately
200,000 eggs per day, which are passed with the feces (2).
-
Unfertilized eggs may be ingested but are not infective. Fertile eggs
embryonate and become infective after 18 days to several weeks (3),
76
-
Depending on the environmental conditions (optimum: moist, warm, shaded
soil). After infective eggs are swallowed (4),
-
the larvae hatch (5),
-
Invade the intestinal mucosa, and are carried via the portal, then systemic
circulation and/or lymphatics to the lungs. The larvae mature further in the
lungs (6) (10 to 14 days),
-
Penetrate the alveolar walls, ascend the bronchial tree to the throat, and are
swallowed (7).
-
Upon reaching the small intestine, they develop into adult worms (8).
-
Between 2 and 3 months are required from ingestion of the infective eggs to
oviposition by the adult female. Adult worms can live 1 to 2 years.
First appearance of eggs in stools is 60–70 days. In larval ascariasis, symptoms occur
4–16 days after infection.
The final symptoms are gastrointestinal discomfort, colic and vomiting, fever, and
observation of live worms in stools.
Some patients may have pulmonary symptoms or neurological disorders during
migration of the larvae.
However there are generally few or no symptoms.
A bolus of worms may obstruct the intestine; migrating larvae may cause pneumonitis
and eosinophilia.
8.8.5 Transmission
The source of transmission is from soil and vegetation on which fecal matter
containing eggs has been deposited.
Ingestion of infective eggs from soil contaminated with human feces or transmission
and contaminated vegetables and water is the primary route of infection.
Intimate contact with pets which have been in contact with contaminated soil may
result in infection, while pets which are infested themselves by a different type of
roundworm can cause infection with that type of worm (Toxocara canis, etc) as
occasionally occurs with groomers.
Transmission also comes through municipal recycling of wastewater into crop fields.
This is quite common in emerging industrial economies, and poses serious risks for
not only local crop sales but also exports of contaminated vegetables.
Transmission from human to human by direct contact is impossible.
8.8.6 Diagnosis
The diagnosis is usually incidental when the host passes a worm in the stool or vomit.
Stool samples for ova and parasites will demonstrate Ascaris eggs. Larvae may be
found in gastric or respiratory secretions in pulmonary disease. B
lood counts may demonstrate peripheral eosinophilia. On X-ray, 15-35 cm long filling
defects, sometimes with whirled appearance (bolus of worms).
77
8.8.7 Symptoms
Patients can remain asymptomatic for very long periods of time.
As larval stages travel through the body, they may cause visceral damage, peritonitis
and inflammation, enlargement of the liver or spleen, toxicity, and pneumonia.
A heavy worm infestation may cause nutritional deficiency;
Other complications, sometimes fatal, include obstruction of the bowel by a bolus of
worms (observed particularly in children) and obstruction of the bile or pancreatic
duct.
Ascaris takes most of its nutrients from the partially digested host food in the intestine.
There is limited evidence that it can also pierce the intestinal mucous membrane and
feed on blood, but this is not its usual source of nutrition. As a result, Ascaris infection
does not produce the anemia associated with some other roundworm infections.
8.8.8 Treatment
Pharmaceutical drugs that are used to kill roundworms are called ascaricides and include:
Mebendazole (Vermox) (C16H13N3O2). Causes slow immobilization and death of the
worms by selectively and irreversibly blocking uptake of glucose and other nutrients
in susceptible adult intestine where helminths dwell. Oral dosage is 100 mg 12 hourly
for 3 days.
Piperazine (C4H10N2.C6H10O4). A flaccid paralyzing agent that causes a blocking
response of ascaris muscle to acetylcholine. The narcotizing effect immobilizes the
worm, which prevents migration when treatment is accomplished with weak drugs
such as thiabendazole. If used by itself it causes the worm to be passed out in the
feces. Dosage is 75 mg/kg (max 3.5 g) as a single oral dose.
Pyrantel pamoate (Antiminth, Pin-Rid, Pin-X) (C11H14N2S.C23H16O6) Depolarizes
ganglionic block of nicotinic neuromuscular transmission, resulting in spastic
paralysis of the worm. Spastic (tetanic) paralyzing agents, in particular pyrantel
pamoate, may induce complete intestinal obstruction in a heavy worm load. Dosage is
11 mg/kg not to exceed 1 g as a single dose.
Albendazole (C12H15N3O2S) A broad-spectrum antihelminthic agent that decreases
ATP production in the worm, causing energy depletion, immobilization, and finally
death. Dosage is 400 mg given as single oral dose (contraindicated during pregnancy
and children under 2 years).
Thiabendazole. This may cause migration of the worm into the esophagus, so it is
usually combined with piperazine.
Hexylresorcinol effective in single dose, mentioned in : Holt, Jr Emmett L, McIntosh
Rustin: Holt's Diseases of Infancy and Childhood: A Textbook for the Use of Students
and Practitioners. Appleton and Co, New York,11th edition
Santonin, more toxic than hexylresorcinol, mentioned in : Holt, Jr Emmett L,
McIntosh Rustin: Holt's Diseases of Infancy and Childhood: A Textbook for the Use
of Students and Practitioners. Appleton and Co, New York,, 11th edition
Oil of chenopodium, more toxic than hexylresorcinol, mentioned in : Holt, Jr Emmett
L, McIntosh Rustin: Holt's Diseases of Infancy and Childhood: A Textbook for the
Use of Students and Practitioners. Appleton and Co, New York, 11th edition
Also, corticosteroids can treat some of the symptoms, such as inflammation.
78
8.8.9 Prevention
Prevention includes: use of toilet facilities; safe excreta disposal; protection of food from dirt
and soil; thorough washing of produce; and hand washing.
Food dropped on the floor should never be eaten without washing or cooking, particularly in
endemic areas. Fruits and vegetables should always be washed thoroughly before
consumption.
8.8.10 Trivia
Ascariasis may result in allergies to shrimp and dustmites due to the shared antigen,
tropomyosin.
Ascaris have an aversion to some general anesthetics and may exit the body,
sometimes through the mouth.
Genus and Species
Ascaris lumbricoides
Common Name
Giant Intestinal Roundworm
Etiologic Agent of:
Ascariasis
Infective stage
Embryonated Egg
Definitive Host
Man
Portal of Entry
Mouth
Mode of Transmission
Ingestion of Embryonated egg through contaminated food
or water
Habitat
Small Intestine
Pathogenic Stage
Adult, Larva
Mode of Attachment
Retention in the mucosal folds using pressure
Mode of Nutrition
Feeding of Chyme
Larva – pneumonitis, Loeffler’s Syndrome;
Pathogenesis
Adult – Obstruction, Liver abscess, Appendicitis. With
Blood-Lung Phase along with Hookworms and
Strongyloides stercoralis.
79
Laboratory diagnosis
Concentration methods and Direct Fecal Smear: Kato-Katz
Treatment
Albendazole, Mebendazole, or Pyrantel pamoate
Diagnostic Feature – Adult Female - prominent genital girdle
Diagnostic Feature – Egg
Coarse mammilated albuminous coating
8.9 TRICHURIASIS
8.9.1 Meaning Trichuriasis.
Trichuriasis is a parasitic disease caused by infection of the large intestine by a
parasite whipworm (Trichuris trichiura).
8.9.2 Causes, incidence, and risk factors
Trichuriasis is common worldwide (in particular among countries with warm, humid
climates) and primarily affects children, who may become infected if they ingest soil
contaminated with whipworm eggs.
The ingested eggs hatch, and the whipworm embeds in the wall of the large intestine
(cecum, colon, rectum).
The main risk factor for infection is ingestion of eggs from soil contaminated with
feces. Some outbreaks have been traced to contaminated vegetables (due to presumed
soil contamination).
8.9.3 Symptoms
Light infestations are frequently asymptomatic (have no symptoms).
Heavy infestations may have bloody diarrhea.
Long-standing blood loss may lead to iron-deficiency anemia.
Rectal prolapse is seen in severe cases.
8.9.4 Signs and tests
Trichuris trichiura egg
A stool ova and parasites exam reveals the presence of typical whipworm eggs.
8.9.5 Treatment
Oral treatment with mebendazole for 3 days is commonly used in symptomatic
infections. Another anti-parasitic agent (albendazole) can be used as an alternative
therapy.
80
8.9.6 Prognosis
Full recovery is expected with treatment.
8.9.7 Complications
In severe cases, dehydration and anemia from bloody diarrhea can occur.
Rarely, rectal prolapse can also occur.
8.9.8 Prevention
Improved facilities for feces disposal have decreased the incidence of whipworm.
Handwashing before food handling,
Avoiding ingestion of soil by thorough washing of food that may have been
contaminated with egg-containing soil are other preventive measures.
8.10 HOOKWORM
8.10.1 What hookworm is
The hookworm is a parasitic nematode worm that lives in the small intestine of its
host, which may be a mammal such as a dog, cat, or human.
Two species of hookworms commonly infect humans, Ancylostoma duodenale and
Necator americanus.
Hookworms are also bilateral, meaning that if cut in half, the worm would be the exact
same on each side.
Necator americanus predominates in the Americas, Sub-Saharan Africa, Southeast
Asia, China, and Indonesia, while A. duodenale predominates in the Middle East,
North Africa, India and (formerly) in southern Europe.
Hookworms are thought to infect more than 600 million people worldwide. The A.
braziliense and A. tubaeforme species infect cats, while A. caninum infects dogs.
Uncinaria stenocephala infects both dogs and cats.
Hookworms are much smaller than the large roundworm, Ascaris lumbricoides, and
the complications of tissue migration and mechanical obstruction so frequently
observed with roundworm infestation are less frequent in hookworm infestation.
The most significant risk of hookworm infection is anemia, secondary to loss of iron
(and protein) in the gut. The worms suck blood voraciously and damage the mucosa.
However, the blood loss in the stools is not visibly apparent.
Ankylostomiasis, alternatively spelled anchylostomiasis and also called
helminthiasis, "miners' anaemia", "tunnel disease", "brickmaker's anaemia"
and "Egyptian chlorosis", is the disease caused by hookworms. It is caused when
hookworms, present in large numbers, produce an iron deficiency anemia by
voraciously sucking blood from the host's intestinal walls. The name is derived from
Greek ancylo "crooked, bent" and stoma "mouth."
Hookworm is a leading cause of maternal and child morbidity in the developing
countries of the tropics and subtropics.
81
In susceptible children hookworms cause intellectual, cognitive and growth
retardation, intrauterine growth retardation, prematurity, and low birth weight among
newborns born to infected mothers.
Hookworm infection is rarely fatal, but anemia can be significant in the heavily
infected individual.
8.10.2 Life cycle
Figure 8.2: Hookworm life cycle
They exist primarily in sandy or loamy soil and cannot live in clay or muck. Rainfall
averages must be more than 1000 mm (40 inches) a year.
Only if these conditions exist can the eggs hatch. Infective larvae of Necator
americanus can survive at higher temperatures, whereas those of Ancylostoma
duodenale are better adapted to cooler climates.
Generally, they live for only a few weeks at most under natural conditions, and die
almost immediately on exposure to direct sunlight or desiccation.
Infection of the host is by the larvae, not the eggs.
While A. duodenale can be ingested, the usual method of infection is through the skin;
this is commonly caused by walking barefoot through areas contaminated with fecal
matter.
The larvae are able to penetrate the skin of the foot, and once inside the body, they
migrate through the vascular system to the lungs, and from there up the trachea, and
are swallowed.
82
They then pass down the esophagus and enter the digestive system, finishing their
journey in the intestine, where the larvae mature into adult worms.
Once in the host gut, Necator tends to cause a prolonged infection, generally 1–5 years
(many die within a year or two of infecting), though some adult worms have been
recorded to live for 15 years or more.
On the other hand, Ancylostoma adults are short lived, surviving on average for only
about 6 months.
However, infection can be prolonged because dormant larvae can be "recruited"
sequentially from tissue "stores" (see Pathology, above) over many years, to replace
expired adult worms.
This can give rise to seasonal fluctuations in infection prevalence and intensity (apart
from normal seasonal variations in transmission).
They mate inside the host, females laying up to 30,000 eggs per day and some 18 to 54
million eggs during their lifetime, which pass out in feces.
Because it takes 5–7 weeks for adult worms to mature, mate and produce eggs, in the
early stages of very heavy infection, acute symptoms might occur without any eggs
being detected in the patient's feces.
This can make diagnosis very difficult.
8.10.3 Incubation Period
The incubation period can vary between a few weeks to many months and is largely
dependent on the number of Hookworm parasites an individual is infected with.
8.10.4 Prevention
The infective larvae develop and survive in an environment of damp dirt, particularly sandy
and loamy soil. They cannot survive in clay or muck. The main lines of precaution are those
dictated by sanitary science:
Do not defecate outside latrines, toilets etc.
Do not use human excrement or raw sewage as manure/fertilizer in agriculture
Deworm pet dogs — canine and feline hookworms rarely develop to adulthood in
humans (Ancylostoma caninum, the common dog hookworm, occasionally develops
into an adult to cause eosinophilic enteritis in people), but their invasive larvae can
cause an itchy rash called cutaneous larva migrans.
8.10.5 Symptoms
There are no specific symptoms or signs of hookworm infection.
They arise from a combination of intestinal inflammation and progressive iron/proteindeficiency anemia.
Larval invasion of the skin might give rise to intense, local itching, usually on the foot
or lower leg, which can be followed by lesions that look like insect bites, can blister
("ground itch"), and last for a week or more.
Animal hookworm larvae on penetrating humans may produce a creeping eruption
called cutaneous larva migrans.
The larvae migrate in tortuous tunnels in between stratum germinativum and stratum
corneum of the skin, causing serpigenous vesicular lesions.
83
With advancing movement of the larvae, the rear portions of the lesions become dry
and crusty.
The lesions are typically intensely pruritic. Coughing, chest pain, wheezing, and fever
will sometimes be experienced by people who have been exposed to very large
numbers of larvae.
Epigastric pains, indigestion, nausea vomiting, constipation, and diarrhea can occur
early or in later stages as well, although gastrointestional symptoms tend to improve
with time.
Signs of advanced severe infection are those of anemia and protein deficiency,
including emaciation, cardiac failure and abdominal distension with ascites.
8.10.6 Diagnosis
Diagnosis depends on finding characteristic worm eggs on microscopic examination of
the stools, although this is not possible in early infection.
The eggs are oval or elliptical, measuring 60 µm by 40 µm, colourless, not bile stained
and with a thin transparent hyaline shell membrane.
When released by the worm in the intestine, the egg contains an unsegmented ovum.
During its passage down the intestine, the ovum develops and thus the eggs passed in
feces have a segmented ovum, usually with 4 to 8 blastomeres.
As the eggs of both Ancylostoma and Necator (and most other hookworm species) are
indistinguishable, to identify the genus, they must be cultured in the lab to allow
larvae to hatch out.
If the fecal sample is left for a day or more under tropical conditions, the larvae will
have hatched out, so eggs might no longer be evident. In such a case, it is essential to
distinguish hookworms from Strongyloides larvae, as infection with the latter has
more serious implications and requires different management.
The larvae of the two hookworm species can also be distinguished microscopically,
although this would not be done routinely, but usually for research purposes. Adult
worms are rarely seen (except via endoscopy, surgery or autopsy), but if found, would
allow definitive identification of the species.
Classification can be performed based on the length of the buccal cavity, the space
between the oral opening and the esophagus: hookworm rhabditoform larvae have
long buccal cavities whereas Strongyloides rhabditoform larvae have short buccal
cavities.[46]
8.10.7 Treatment
The hookworm can be treated with local cryotherapy when it is still in the skin.
Albendazole is effective both in the intestinal stage and during the stage the parasite is
still migrating under the skin.
In case of anemia, iron supplementation can cause relief symptoms of iron deficiency
anemia. However, as red blood cell levels are restored, shortage of other essentials
such as folic acid or vitamin B12 may develop, so this might also be supplemented.
The most common treatment for Hookworm is Benzimidazoles (BZAs), specifically
albendazole and mebendazole. BZAs kill adult worms by binding to the nematode’s
beta-tubulin and subsequently inhibiting microtubule polymerization within the
parasite.
In certain circumstances, levamisole and pyrantel pamoate may be used.
84
Also of note is that the World Health Organization does recommend anthelmintic
treatment in pregnant women after the first trimester. It is also recommended that if
the patient also suffers from anemia that ferrous sulfate (200 mg) be administered
three times daily at the same time as anthelmintic treatment; this should be continued
until hemoglobin values return to normal which could take up to 3 months.
8.10.8 Trivia
Genus and Species
Necator americanus
Common Name
New world hookworm, American
Old world hookworm
murderer
Etiologic Agent of:
Necatoriasis, Uncinariasis
Ancylostomiasis, Wakana disease
Infective stage
Filariform larva
Filariform larva
Definitive Host
Man
Man
Portal of Entry
Usually via skin penetration Usually via ingestion rather than
rather than ingestion
skin penetration
Mode
Transmission
of
Ancylostoma duodenale
Skin > Mouth
Mouth > Skin
Habitat
Small Intestine (jejunum, ileum)
Small
Intestine
jejunum)
Pathogenic Stage
L3 Larva
L3 Larva
Maturation time in
49-56
host (days)
Mode
Attachment
53
of Oral attachment to mucosa by
Same
sucking
Mode of Nutrition
Sucking and Ingesting of blood
Pathogenesis
Larva – ground / dew itch,
Same
creeping eruption
85
Same
(duodenum,
Adult – IDA Microcytic,
Hypochromic Anemia
Laboratory
diagnosis
Concentration
methods
Direct Fecal Smear
and
Treatment
Albendazole, Mebendazole, or
Same
Pyrantel Pamoate
Length of adult
5-9 for males; 9-11 for females
hookworm (mm)
Shape
Same
8-11 for males; 10-13 for females
Head
curved
opposite
to
curvature of body, giving a Head continuous in same direction
hooked appearance to anterior as the body
end
Egg output per
female worm per 5,000-10,000
day
10,000-25,000
Blood loss per
0.03
worm per day (ml)
0.15-0.23
Temperature
at
which 90% of eggs 20-35
hatch (C)
15-35
cutting
Diagnostic Feature Semi-lunar
Bipartite dorsal ray
- Adult
plate;
Diagnostic Feature
In Morula
- Egg
Male – Tripartite dorsal ray
Same
8.12 Summary
Helminthiasis is infestation with one or more intestinal parasitic worms roundworms (Ascaris
lumbricoides), whipworms (Trichuris trichiura), or hookworms (Necator americanus and
Ancylostoma duodenale)).
86
Infected people excrete helminth eggs in their faeces, which then contaminate the soil in areas
with inadequate sanitation. Other people can then be infected by ingesting eggs or larvae in
contaminated food, or through penetration of the skin by infective larvae in the soil
(hookworms).
Infestation can cause morbidity, and sometimes death, by compromising nutritional status,
affecting cognitive processes, inducing tissue reactions, such as granuloma, and provoking
intestinal obstruction or rectal prolapse.
Control of helminthiasis is based on drug treatment, improved sanitation and health education.
8.12 Self-Test Questions
i)
ii)
iii)
iv)
v)
Describe what helminthiasis is.
Explain the causes of helminthiasis.
Explain complications resulting from helminthiasis.
Describe the prevation and control of helminthiasis.
Describe various infections resulting from helminthiasis
87
LECTURE NINE
TROPICAL DISEASES: DIARRHOEA AND TYPHOID FEVER.
9.1 Introduction
Welcome to the ninth lecture in our course. In this lecture we will cover diarrhea and typhoid
fever. The broader objective of this lecture is to enable you to describe diarrhea and typhoid
fever.
9.2 Objectives
By end of this lecture you should be able to:
i) Describe what diarrhea and typhoid fever are.
ii) Explain the causes, transmission, diagnosis, signs and
symptoms, prevention and control of diarrhoea and typhoid
fever.
9.3 DIARRHOEA
9.3.1 What diarrhoea is
Diarrhoea is the passage of loose or liquid stools more frequently than is normal for
the individual.
It is passing of more than three loose motions in a day or 24hours
It has been further classified an acute diarrhoea i.e. lasting for less than 21 days and
chronic diarrhoea, lasting beyond 21 days.
While chronic diarrhoea is responsible for the serious problems of malnutrition, acute
diarrhoea is responsible for death due to dehydration.
It is primarily a symptom of gastrointestinal infection. Depending on the type of
infection, the diarrhoea may be watery (for example in cholera) or passed with blood
(in dysentery for example).
Diarrhoea due to infection may last a few days, or several weeks, as in persistent
diarrhoea.
Severe diarrhoea may be life threatening due to fluid loss in watery diarrhoea,
particularly in infants and young children, the malnourished and people with impaired
immunity.
The impact of repeated or persistent diarrhoea on nutrition and the effect of
malnutrition on susceptibility to infectious diarrhoea can be linked in a vicious cycle
amongst children, especially in developing countries.
Diarrhoea is also associated with other infections such as malaria and measles.
Chemical irritation of the gut or non-infectious bowel disease can also result in
diarrhoea.
88
9.3.2 Cause
Diarrhoea is a symptom of infection caused by a host
i)
ii)
iii)
Bacteria e.g. Escherichia coli, Shigella, Salmonella, Vibro cholerae,
Choleral El Tor, campylobacter jejuni, staphy lococcus.
Virus e.g. Rota virus, Adenovirus, Astrovirus, Calcivirus, Nov fork
group of viruses
Parasites e.g. Entameoba hystolica, Giardia Lamblin, stronglyoids,
Trichuris.
9.3.3 Transmission
Most of these organisms can be spread by contaminated water. It is more common
when there is a shortage of clean water for drinking, cooking and cleaning and basic
hygiene is important in prevention.
Water contaminated with human faeces for example from municipal sewage, septic
tanks and latrines is of special concern. Animal faeces also contain microorganisms
that can cause diarrhoea.
Diarrhoea can also spread from person to person
i.
ii.
iii.
Through direct contact, aggravated by poor personal hygiene.
Food is another major cause of diarrhoea when it is prepared or stored
in unhygienic conditions.
Water can contaminate food during irrigation, and fish and seafood
from polluted water may also contribute to the disease.
9.3.4 Distribution
The infectious agents that cause diarrhoea are present or are sporadically introduced
throughout the world.
Diarrhoea is a rare occurrence for most people who live in developed countries where
sanitation is widely available, access to safe water is high and personal and domestic
hygiene is relatively good.
World-wide around 1.1 billion people lack access to improved water sources and 2.4
billion have no basic sanitation.
Diarrhoea due to infection is widespread throughout the developing world. In
Southeast Asia and Africa, diarrhoea is responsible for as much as 8.5% and 7.7% of
all deaths respectively.
9.3.5 Scope of the Problem
Diarrhoea occurs world-wide and causes 4% of all deaths and 5% of health loss to
disability.
It is most commonly caused by gastrointestinal infections which kill around 2.2
million people globally each year, mostly children in developing countries.
Each year there are approximately 4 billion cases of diarrhoea worldwide.
The use of water in hygiene is an important preventive measure but contaminated
water is also an important cause of diarrhoea.
89
Cholera and dysentery cause severe, sometimes life threatening forms of diarrhoea.
9.3.6 Signs and symptoms
It depends upon the severity of the disease.
When diarrhea is severe, signs of dehydration occur quickly especially in children.
1. Dehydration
i.
ii.
iii.
iv.
v.
vi.
vii.
Little to extreme loss of subcutaneous fat
Up to 50% total body weight loss.
Urinary output decreases
Poor skin turgor dry skin and dry mouth
Sunken fontanelles and eyes
Low BP and high pulse.
Collapse imminent
2. Behavior changes
i.
ii.
iii.
iv.
v.
vi.
Irritability
Restlessness
Weakness
Pallor
Extreme prostration
Stupor and convulsions
3. Respiration Rapid i.e. hyperpnoea
4. Stools
i.
ii.
iii.
Loose and fluid consistency
Greenish or yellow green colour
May contain mucus or blood
5. Vomiting mild and intermittent to severe vomiting
6. Fever low grade to 40.1ºc (100ºF)
7. Anorexia
9.3.7 Diagnosis
i. History and information is collected from patient including:
a) Diarrhea e.g. duration, frequency, appearance, consstency, colour, smell,
presence or absence of blood/mucus.
b) Vomiting
c) Thirst normal or more than normal
d) Urine out put
ii. Look and examine the general conditions of the patient for signs of dehydration
iii. Collect the specimen of stool for laboratory diagnosis
90
9.3.8 Treatment and Interventions
Key measures to treat diarrhoea include:
Giving more fluids than usual, including oral rehydration salts solution, to prevent
dehydration.
Continue feeding.
Consulting a health worker if there are signs of dehydration or other problems.
The intervention measures comprise the following:
a) Oral Rehydration therapy
-
It is a life-saving measure to combat dehydration.
It should be started forthwith to prevent the further damages and prevent the
death.
It is envisaged in 3 stages
i. Managing diarrhea situation with home made available liquids
ii. Oral rehydration salts be encouraged to combat dehydration
iii. Primary health centres and hospitals will entreat with IV therapy in severe
cases.
Preparation of ORS at home
i.
ii.
iii.
iv.
v.
vi.
Take half a litre of clean drinking water
Add 2 finger pinch of salt, stir it well.
Taste a spoon full of the solution, it should not be more salty than tears
Add a large fistful of sugar
Stir the mixture well until sugar dissolve
Give ¼ to ½ cup (50-100ml) of this mixture after every loose motion to
a child less than 2 years of age and twice the amount in children above
2 years of age. Give small sips of the drink if the child vomits
Preparation of ORS with ORS packet
i.
ii.
iii.
iv.
v.
Take litre of clean drinking water
Obtain an ORS packet
Pour the water and the powder from the packet into a large clean vessel
Stir it well till it dissolves. There will be about 5 glasses of the drink.
Give ¼ to ½ cup (50-100ml) of this mixture after every loose motion to
a child less than 2 years of age and twice the amount in children above
2 years of age. Make fresh drink every day. The solution should be
used within 24 hours. Give small amounts of sip by sip of the drink
b) Appropriate feeding
During episodes of diarrhea normal food intake like coconut water, rice water,
dal water, mashed ripe banana, weak tea including breast-feeding.
91
c) Appropriate Drugs
For bacterial infection ampicillin, chloramphenicol, genticin and tetracycline are used.
Symptomatic treatment for fever, vomiting etc. is done. For protozoal infection
metronidazole can be used.
9.3.9 Control and prevention
Key measures to reduce the number of cases of diarrhoea include:
Access to safe drinking water.
Improved sanitation.
Good personal and food hygiene.
Health education about how infections spread.
9.4 TYPHOID FEVER
9.4.1 What typhoid fever is
Typhoid fever is a bacterial infection characterized by diarrhoea, systemic disease, and a rashmost commonly caused by the bacteria Salmonella typhi (S. typhi).
9.4.2 Causes and transmission
The bacteria that causes typhoid fever -- S. typhi -- spreads through contaminated food, drink,
or water. If you eat or drink something that is contaminated, the bacteria enters your body,
and goes into your intestines, and then into your bloodstream, where it can travel to your
lymph nodes, gallbladder, liver, spleen, and other parts of the body.
A few people can become carriers of S. typhi and continue to release the bacteria in their
stools for years, spreading the disease.
Typhoid fever is common in developing countries.
9.4.3 Symptoms
Early symptoms include
-
fever
general ill-feeling, and
Abdominal pain.
A high (over 103 degrees) fever and severe diarrhea occur as the disease gets
worse.
Some people with typhoid fever develop a rash called "rose spots," which are
small red spots on the belly and chest.
Other symptoms that occur include:
Abdominal tenderness
92
Agitation
Bloody stools
Chills
Confusion
Difficulty paying attention (attention deficit)
Delirium
Fluctuating mood
Hallucinations
Nosebleeds
Severe fatigue
Slow, sluggish, lethargic feeling
Weakness
9.4.4 Diagnosis
-
A complete blood count (CBC) is taken and will show a high number of white
blood cells.
A blood culture during first week of the fever can show S. typhi bacteria
Other tests that can help diagnose this condition include:
Culture Stool culture
ELISA urine test to look for the bacteria that causes Typhoid fever
Platelet count (platelet count will be low)
Fluorescent antibody study to look for substances specific to Typhoid bacteria
9.4.5 Treatment
Fluids and electrolytes may be given through a vein (intravenously). Appropriate antibiotics
are given to kill the bacteria. There are increasing rates of antibiotic resistance throughout the
world, so your health care provider will check current recommendations before choosing an
antibiotic.
9.4.6 Prognosis
Symptoms usually improve in 2 to 4 weeks with treatment. The outcome is likely to be good
with early treatment, but becomes poor if complications develop.
Symptoms may return if the treatment has not completely cured the infection.
9.4.7 Possible Complications
Intestinal haemorrhage (severe GI bleeding)
Intestinal perforation
Kidney failure
Peritonitis
93
9.4.8 Prevention
Vaccines are recommended for those travelling to epidemic areas.
Immunization is not always completely effective and at-risk travelers should drink only boiled
or bottled water and eat well cooked food. Experimentation with an oral live attenuated
typhoid vaccine is now underway and appears promising.
Adequate water treatment, waste disposal, and protection of food supply from contamination
are important public health measures. Carriers of typhoid must not be allowed to work as food
handlers.
Health education is also very important.
9.6 Summary
Diarrhoea is the passage of loose or liquid stools more frequently than is normal for
the individual.
While chronic diarrhoea is responsible for the serious problems of malnutrition, acute
diarrhoea is responsible for death due to dehydration.
It is primarily a symptom of gastrointestinal infection. Depending on the type of
infection, the diarrhoea may be watery (for example in cholera) or passed with blood
(in dysentery for example).
Severe diarrhoea may be life threatening due to fluid loss in watery diarrhoea,
particularly in infants and young children, the malnourished and people with impaired
immunity.
It is caused Bacteria, Virus, Parasites
Key measures to treat diarrhoea include:
-
Giving more fluids than usual, including oral rehydration salts solution, to
prevent dehydration.
Continue feeding.
Consulting a health worker if there are signs of dehydration or other
problems.
The intervention measures comprise the following:
Key measures to reduce the number of cases of diarrhoea include:
-
Access to safe drinking water.
Improved sanitation.
Good personal and food hygiene.
Health education about how infections spread.
Typhoid fever is a bacterial infection characterized by diarrhoea, systemic disease, and
a rash- most commonly caused by the bacteria Salmonella typhi (S. typhi).
The bacteria that causes typhoid fever -- S. typhi -- spreads through contaminated food,
drink, or water. If you eat or drink something that is contaminated, the bacteria enters
94
your body, and goes into your intestines, and then into your bloodstream, where it can
travel to your lymph nodes, gallbladder, liver, spleen, and other parts of the body
There are generally four lines of defence against typhoid fever:
o Control of sanitation
o Control of reservoir
o Immunization
o Health Education
Dengue fever is a disease caused by a family of viruses that are transmitted by
mosquitoes.
Symptoms such as headache, fever, exhaustion, severe joint and muscle pain, swollen
glands (lymphadenopathy), and rash. The presence (the "dengue triad") of fever, rash,
and headache (and other pains) is particularly characteristic of dengue fever.
Dengue is prevalent throughout the tropics and subtropics.
Because dengue fever is caused by a virus, there is no specific medicine or antibiotic
to treat it. For typical dengue fever, the treatment is purely concerned with relief of the
symptoms (symptomatic).
The prevention of dengue fever requires control or eradication of the mosquitoes
carrying the virus that causes dengue.
There is currently no vaccine available for dengue fever.
9.7 Self-test questions
i) Describe what diarrhoea, typhoid fever and dengue fever are.
ii) Explain the factors that contribute to the infections among your community
members.
iii) Describe how the infections can be controlled and prevented.
iv) List the signs and symptoms for diarrhoea, typhoid fever, and dengue fever.
95
9.8 Further reading
Bhutta ZA. Typhoid fever. In: Rakel P, Bope ET, eds. Conn’s Current Therapy 2008. 60th ed.
Philadelphia, Pa: Saunders Elsevier; 2008:chap 48.
Canada. Public Health Agency of Canada. "Dengue in South East Asia." Aug. 23, 2007.
http://www.phac-aspc.gc.ca/tmp-pmv/2007/dengue070823_e.html.
"Dengue
Fever
in
Key
West."
Florida
Department
of
Health.
http://www.doh.state.fl.us/Environment/medicine/arboviral/Dengue_FloridaKeys.html.
Kaye KS, Kaye D. Salmonella infections (including typhoid fever). In: Goldman L, Ausiello
D, eds. Cecil Medicine. 23rd ed. Philadelphia, Pa: Saunders Elsevier; 2007: chap 329.
Switzerland. World Health Organization. "Planning Social Mobilization and Communication
for Dengue Fever Prevention and Control."
http://www.who.int/tdr/publications/publications/pdf/planning_dengue.pdf.
Switzerland. World Health Organization. "Vector-Borne Viral Infections."
http://www.who.int/vaccine_research/diseases/vector/en/index.html.
United States. Centers for Disease Control and Prevention. "Dengue." May 20, 2010.
http://www.cdc.gov/Dengue/.
United States. Centers for Disease Control and Prevention. "Locally Acquired Dengue -- Key
West, Florida, 2009-2010." Morbidity and Mortality Weekly Report 59.19 May 21, 2010: 577581. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5919a1.htm.
WHO (2000) Global Water Supply and Sanitation Assessment. World Health Organization.
Geneva
World Health Report 2000, World Health Organization (WHO), Geneva
96
LECTURE TEN
TROPICAL DISEASES: POLIOMYELITIS, MEASLES, DIPHTHERIA,
WHOOPING COUGH
10.1 Introduction
Welcome to the tenth lecture in our course. In this lecture we will cover poliomyelitis,
measles, diphtheria and whooping cough. The broader objective of this lecture is to enable
you to describe poliomyelitis, measles, diphtheria and whooping cough..
10.2 Objectives
By end of this lecture you should be able to:
i) Describe what poliomyelitis, measles, diphtheria and whooping
cough are.
ii) Explain causes, transmission, diagnosis, signs and symptoms,
treatment, control and prevention measures for poliomyelitis,
measles, diphtheria and whooping cough.
10.3 POLIOMYELITIS
10.3.1 What poliomyelitis is
Poliomyelitis is a viral disease that can affect nerves and can lead to partial or full paralysis
10.3.2 Causes
Poliomyelitis is a disease caused by infection with the poliovirus. The virus spreads by direct
person-to-person contact, by contact with infected mucus or phlegm from the nose or mouth,
or by contact with infected feces.
The virus enters through the mouth and nose, multiplies in the throat and intestinal tract, and
then is absorbed and spread through the blood and lymph system.
10.3.3 Incubation period
The time from being infected with the virus to developing symptoms of disease (incubation)
ranges from 5 - 35 days (average 7 - 14 days).
Risks include:
Lack of immunization against polio and then exposure to polio
97
Travel to an area that has experienced a polio outbreak
In areas where there is an outbreak, those most likely to get the disease include children,
pregnant women, and the elderly.
10.3.4 Distribution
Between 1840 and the 1950s, polio was a worldwide epidemic. Since the development of
polio vaccines, the incidence of the disease has been greatly reduced. Polio has been wiped
out in a number of countries. There have been very few cases of polio in the Western
hemisphere since the late 1970s.
Outbreaks still occur in the developed world, usually in groups of people who have not been
vaccinated. Polio often occurs after someone travels to a region where the disease is common.
Thanks to a massive, global, vaccination campaign over the past 20 years, polio exists only in
a few countries in Africa and Asia.
10.3.5 Symptoms
There are three basic patterns of polio infection: subclinical infections, nonparalytic, and
paralytic. Approximately 95% of infections are subclinical infections, which may not have
symptoms.
a) Subclinical Infection
General discomfort or uneasiness (malaise)
Headache
Red throat
Slight fever
Sore throat
Vomiting
People with subclinical polio infection might not have symptoms, or their symptoms may last
72 hours or less.
Clinical poliomyelitis affects the central nervous system (brain and spinal cord), and is
divided into non-paralytic and paralytic forms. It may occur after recovery from a subclinical
infection.
b) Non-paralytic Poliomyelitis
Back pain or backache
Diarrhea
Excessive tiredness, fatigue
Headache
Irritability
Leg pain (calf muscles)
Moderate fever
98
Muscle stiffness
Muscle tenderness and spasm in any area of the body
Neck pain and stiffness
Pain in front part of neck
Pain or stiffness of the back, arms, legs, abdomen
Skin rash or lesion with pain
Vomiting
Symptoms usually last 1 - 2 weeks.
c) Paralytic Poliomyelitis
Fever 5 - 7 days before other symptoms
Abnormal sensations (but not loss of sensation) in an area
Bloated feeling in abdomen
Breathing difficulty
Constipation
Difficulty beginning to urinate
Drooling
Headache
Irritability or poor temper control
Muscle contractions or muscle spasms in the calf, neck, or back
Muscle pain
Muscle weakness, asymmetrical (only on one side or worse on one side)
o Comes on quickly
o Location depends on where the spinal cord is affected
o Worsens into paralysis
Sensitivity to touch; mild touch may be painful
Stiff neck and back
Swallowing difficulty
10.3.6 Diagnosis
The health care provider may find signs of meningeal irritation (similar to meningitis,
such as stiff neck or back stiffness with difficulty bending the neck. The person also
might have difficulty lifting the head or lifting the legs when lying flat on the back, and
their reflexes might be abnormal.
Tests include:
Routine Cerebrospinal Fluid (CSF) examination
Test for levels of antibodies to the polio virus
Viral cultures of throat washings, stools, or Cerebrospinal Fluid (CSF)
10.3.7 Treatment
The goal of treatment is to control symptoms while the infection runs its course.
People with severe cases may need lifesaving measures, especially breathing help.
99
Symptoms are treated based on how severe they are. Treatments include:
Antibiotics for urinary tract infections
Medications (such as bethanechol) for urinary retention
Moist heat (heating pads, warm towels) to reduce muscle pain and spasms
Pain killers to reduce headache, muscle pain, and spasms (narcotics are not usually
given because they increase the risk of breathing difficulty)
Physical therapy, braces or corrective shoes, or orthopedic surgery to help recover
muscle strength and function
10.3.8 Prognosis
What to expect depends on the form of the disease (subclinical, nonparalytic, or
paralytic) and the site affected. If the spinal cord and brain are not involved, which is
the case more than 90% of the time, complete recovery is likely.
Brain or spinal cord involvement is a medical emergency that may result in paralysis
or death (usually from respiratory difficulties).
Disability is more common than death. Infection high in the spinal cord or in the brain
increases the risk of breathing problems.
10.3 9 Possible Complications
Aspiration pneumonia
Cor pulmonale
High blood pressure
Kidney stones
Lack of movement
Lung problems
Myocarditis
Paralytic ileus (loss of intestinal function)
Permanent muscle paralysis, disability, deformity
Pulmonary edema
Shock
Urinary tract infections
Post-polio syndrome is a complication that develops in some patients, usually 30 or more
years after their initial infection. Weakness may get worse in muscles that were previously
weakened. Weakness may also develop in muscles that previously were thought not to be
affected.
10.3.10 Prevention
Polio immunization (vaccine) effectively prevents
(immunization is over 90% effective).
100
poliomyelitis
in
most
people
10.4 MEASLES
10.4.1 What Measles is
Measles is best known for causing a rash in childhood, but measles can affect other parts of
the body and sometimes occurs in adults. Vaccination has significantly reduced the number of
cases in the United States, although isolated outbreaks continue to occur.
There are two types of measles, each caused by a different virus. Although both produce a
rash and fever, they are really different diseases:
The rubeola virus causes "red measles," also known as "hard measles" or just
"measles." Although most people recover without problems, rubeola can lead to
pneumonia or inflammation of the brain (encephalitis).
The rubella virus causes "German measles," also known as "three-day measles." This
is usually a milder disease than red measles. However, this virus can cause significant
birth defects if an infected pregnant woman passes the virus to her unborn child.
10.4.2 Measles Causes
Both the rubeola and rubella viruses are spread through the respiratory route. This
means they are contagious through coughing and sneezing. In fact, the rubeola virus is
one of the most contagious viruses known to man. As a result, it can spread rapidly in
a susceptible population. Infected people carry the virus in their respiratory tract
before they get sick, so they can spread the disease without being aware of it.
If people are immune to the virus (either through vaccination or by having had
measles in the past), they cannot get the disease caused by that virus. For example,
someone who had rubeola as a child would not be able to get the disease again.
Remember that rubella and rubeola are different viruses. An infection with one of
these viruses does not protect against infection with the other.
10.4.3 Measles Symptoms
10.4.3.1 Rubeola ("red measles" or "hard measles")
Symptoms appear about 10-14 days after a person is infected with the rubeola virus. This is
called the incubation period. During this period, the virus is multiplying. Symptoms occur in
two phases.
The early phase begins with these symptoms:
o Fever
o A run-down feeling
o Cough
o Red eyes (conjunctivitis)
o Runny nose
o Loss of appetite
The red measles rash develops from two to four days later.
o The rash usually starts on the face, spreading to the trunk and then to the arms
and legs.
101
o
o
o
o
o
The rash is initially small red bumps that may blend into each other as more
appear. From a distance, the rash often looks uniformly red.
People with measles may develop small grayish spots on the inside of the
cheek, called "Koplik spots."
The rash is usually not itchy, but as it clears up, the skin may shed (this looks
like skin that is peeling after sunburn).
Although red measles is usually a mild disease, a few serious complications
may occur. Red measles makes patients more vulnerable to pneumonia and
bacterial ear infections. Pneumonia as a complication of measles is especially
serious in infants and is responsible for most deaths in this age group.
Inflammation of the brain (encephalitis) occurs about once in every thousand
cases and is a serious complication that can be fatal.
Red measles is particularly severe in patients with weakened immune systems,
including people who are malnourished or have HIV.
10.4.3.2 Rubella ("German measles")
German measles causes milder symptoms than red measles. The incubation period between
getting the virus and getting sick is 10 days to two weeks.
Initially, some people experience fatigue, low-grade fever, headache, or red eyes
several days before the rash appears. These symptoms are more common in adults
than in children.
Swollen, tender lymph nodes may occur in the back of the neck.
The rash is light red to pink. It starts as individual spots which may merge together
over time. The rash usually starts on the face and moves down to the trunk.
The rash does not usually itch, but as it clears up, the skin may shed.
Adult women who get rubella may get painful joints for days to weeks after the
infection. This affects the hands, wrists, and knees.
Symptoms may be so mild that they are not even noticed, especially in children. Most
symptoms resolve in a few days, but swollen lymph nodes may persist for a few
weeks.
The most feared complication of rubella is "congenital rubella," which occurs when an
infected pregnant woman passes the virus to her unborn child. Among other problems
and birth defects, affected infants may have cataracts, heart defects, hearing
impairment, and learning disabilities. The risk of transmission is highest early in
pregnancy. The virus may also cause miscarriage or stillbirth.
10.4.4 Diagnosis
Depending on the symptoms, the doctor may diagnose measles based on history and
physical exam alone.
In questionable cases, the doctor can perform specialized blood tests to help with the
diagnosis, but these tests usually are not needed.
Blood tests can also be used to see if a person is immune to measles.
102
10.4.5 Measles Treatment
10.4.5.1 Self-Care at Home
Although there is no cure for measles, there are steps that can make the disease more
tolerable. These include the following:
Get plenty of rest.
Sponge baths with lukewarm water may reduce discomfort due to fever.
Drink plenty of fluids to help avoid dehydration.
A humidifier or vaporizer may ease the cough.
Pain relievers and fever reducers such as acetaminophen (Tylenol, Liquiprin Drops,
and other brands) and ibuprofen (Advil, Motrin and other brands) can help with
symptoms when used according to directions. Remember never to give aspirin to
children or teenagers because it may cause a disease known as Reye syndrome.
10.4.5.2 Medical Treatment
There is no specific treatment or cure for measles. Children should stay at home and
out of school until they are cleared to return by their health-care provider.
10.4.6 Prevention
Because of widespread vaccination of children, both kinds of measles occur much less often
than in the past.
The most effective way to prevent measles is through immunization.
o Children should routinely receive the measles-mumps-rubella (MMR) vaccine
according to a published immunization schedule. This vaccine protects against
both red measles and German measles. Vaccination (or written refusal) is
required for entry into school.
o Doctors usually give the first dose of the measles immunization at 12-15
months of age.
o Doctors give a second dose of the immunization when the child is 4 to 6 years
old.
o Although most children tolerate the vaccine well, a few may develop fever and
even a rash from five to 12 days after the immunization. Adult women who get
the vaccine may notice short-term aching in their joints.
o The vaccine is about 95% effective in preventing measles of either type. That
means that a small number of people who get the vaccine may still be able to
get measles.
o The vaccine should not be used in people with egg allergies.
o Rarely, the measles vaccine can cause a measles-like illness. This is most
common in people with weak immune systems, such as those with advanced
HIV or those on chemotherapy. In such patients, the risk of vaccination should
be balanced carefully against the risk of getting measles.
o Women who may become pregnant should have a blood test to be sure they are
immune to rubella ("German measles").
Both types of measles are still common in areas that do not offer immunization and in
people who have not been immunized.
103
As with all other contagious illnesses, covering the mouth when coughing or sneezing
and good hand-washing practices will help prevent the spread of the diseases.
A special immunization -- immune globulin -- may be necessary for certain high-risk
people after they are exposed to measles. These include children younger than 1 year,
children with weakened immune systems, and pregnant women. If you have been
exposed to measles, contact your physician to determine if you need immune globulin.
10.4.7 Prognosis
Measles of either type usually clears up on its own in seven to 10 days. Once a person
has had a case of the measles, they are almost always immune for life.
As discussed above, complications are rare, but may be serious. This is the reason why
vaccination is so universally recommended.
10.5 DIPHTHERIA
What is diphtheria?
What is the history of diphtheria?
What causes diphtheria?
How is diphtheria transmitted?
What are the signs and symptoms of diphtheria?
How is diphtheria diagnosed?
What is the treatment for diphtheria
What are the complications of diphtheria?
How is diphtheria prevented?
10.5.1 What diphtheria is
Diphtheria is an infectious disease caused by the bacterium Corynebacterium
diphtheriae.
This disease primarily affects the mucous membranes of the respiratory tract
(respiratory diphtheria), although it may also affect the skin (cutaneous diphtheria) and
lining tissues in the ear, eye, and the genital areas.
10.5.2 History of diphtheria
Throughout history, diphtheria was a leading cause of death among children, and it was once
referred to as the "strangling angel of children." Through the ages, several epidemics struck
Europe, and even the American colonies were affected by an outbreak in the 18th century.
Most recently, in the 1990s, large outbreaks of diphtheria occurred in Russia and in the former
independent states of the Soviet Union.
The diphtheria bacterium was first identified in the 1880s. In the 1890s, the antitoxin against
diphtheria was developed, with the first vaccine being developed in the 1920s. With the
development and administration of the diphtheria vaccine, the incidence of diphtheria has
decreased significantly. Furthermore, whereas diphtheria primarily affected younger children
in the prevaccination era, an increasing proportion of cases today occur in unvaccinated or
inadequately immunized adolescents and adults.
104
10.5.3 Causes of diphtheria
Diphtheria is caused by toxin-producing strains of the gram-positive bacillus
Corynebacterium diphtheriae.
There are four biotypes of the bacterium:
-
gravis,
mitis,
intermedius, and
belfanti
Each differs in the severity of disease it produces. Non-toxigenic strains are usually
responsible for less severe cutaneous diphtheria.
The signs and symptoms of respiratory diphtheria are caused by the bacterium's ability
to cause a localized inflammatory reaction of the cells lining the upper respiratory
tract.
In certain cases, the disease can become more severe and widespread, and it can
involve other organs of the body as well.
10.5.4 Transmission
Diphtheria is transmitted to close contacts via airborne respiratory droplets or by direct
contact with nasopharyngeal secretions or skin lesions.
Rarely, it can be spread by objects contaminated by an infected person.
Overcrowding and poor living conditions can further contribute to the spread of
diphtheria.
Humans are the only known reservoir of Corynebacterium diphtheriae.
Infected individuals may develop symptoms of diphtheria, or they may become
carriers of the bacteria with no symptoms (asymptomatic carriers).
These asymptomatic carriers can serve as reservoirs for active infection and may
transmit the disease to other individuals.
10.5.5 Signs and symptoms of diphtheria
The symptoms of respiratory diphtheria usually begin after a two- to five-day incubation
period. Symptoms of respiratory diphtheria may include the following:
sore throat,
fever,
malaise,
hoarseness,
difficulty swallowing, or
Difficulty breathing.
With the progression of respiratory diphtheria, the infected individual may also develop an
adherent gray membrane (pseudomembrane) forming over the lining tissues of the tonsils
and/or nasopharynx. Individuals with severe disease may also develop neck swelling and
enlarged neck lymph nodes, leading to a "bull-neck" appearance. Extension of the
105
pseudomembrane into the larynx and trachea can lead to obstruction of the airway with
subsequent suffocation and death.
The dissemination of diphtheria toxin can also lead to systemic disease, causing complications
such as inflammation of the heart (myocarditis) and neurologic problems such as paralysis of
the soft palate, vision problems, and muscle weakness.
Cutaneous diphtheria is characterized by a non-healing skin ulcer covered by a gray-brown
membrane. It is typically a localized infection that is rarely associated with systemic
complications.
10.5.6 Diagnosis
The diagnosis of diphtheria is confirmed by isolation of the bacterium
Corynebacterium diphtheriae.
Diagnostic tests to isolate the bacterium involve obtaining cultures from the nose and
throat in any individual suspected of having diphtheria, as well as their close contacts.
It is also important to determine whether or not the isolate is capable of producing
diphtheria toxin, and this can be accomplished by testing in specialized laboratories.
Finally, determining the patient's antibody levels to diphtheria toxin can also be
helpful for evaluating the probability of the diagnosis of diphtheria and the potential
for severe illness.
Other tests, such as ECG, imaging studies, and blood work can also help assess the
extent of involvement of the disease.
10.5.7 Treatment for diphtheria
If diphtheria is suspected in a patient, prompt treatment should be undertaken even
before confirmatory lab results are available.
Diphtheria antitoxin is the mainstay of therapy. It neutralizes circulating diphtheria
toxin and reduces the progression of the disease. The effectiveness of diphtheria
antitoxin is greatest if it is administered early in the course of the disease.
Antibiotics should also be administered as soon as possible to patients with suspected
diphtheria. Antibiotics help eradicate the bacteria, thereby stopping toxin production,
and they also help to prevent transmission of diphtheria to close contacts.
Penicillin and erythromycin are the recommended antibiotics.
Asymptomatic carriers, as well as all close contacts potentially exposed to diphtheria,
also require antibiotic treatment.
Supportive measures, such as inserting a breathing tube (intubation), may be necessary
if the patient cannot breathe on their own or if there is the potential for airway
obstruction.
Potential cardiac and neurologic complications also need to be closely followed and
addressed in consultation with the proper specialist.
106
10.5.8 Complications of diphtheria
The potential complications of diphtheria may include the following:
Cardiac (inflammation of the heart, heart valve infection, heart rhythm disturbances,
and congestive heart failure),
Neurologic (muscle paralysis, muscle weakness, and vision problems),
Infectious (lung infection, blood infection, and bone infection), and
Death.
For respiratory diphtheria, the fatality rate is 10%-15%, although it may be higher in patients
less than 5 years of age and older than 40 years of age. Airway obstruction and cardiac
complications are the most common causes of death.
10.9 Prevention of diphtheria
The prevention of diphtheria is best achieved through universal immunization with
diphtheria toxoid-containing vaccines.
Immunization for infants and children consists of five DTaP vaccinations generally
given at ages 2, 4, and 6 months, with the fourth dose being administered between 1518 months, and the fifth dose at ages 4-6 years.
At age 11-12 years, children should receive a single Tdap vaccination if they have
completed the recommended childhood vaccination schedule.
Because immunity wanes over time, subsequent booster immunization is required
every 10 years thereafter to maintain protective antibody levels.
Travelers to areas where diphtheria is endemic should review and update their vaccinations as
necessary.
10.5.10 Conclusion
Diphtheria is an infectious disease caused by the bacterium Corynebacterium
diphtheriae.
Diphtheria is primarily transmitted via airborne respiratory droplets or by direct
contact with secretions from infected people.
The symptoms of diphtheria include sore throat, fever, malaise, difficulty swallowing,
and difficulty breathing.
Diphtheria is treated with both antitoxin and antibiotics.
Diphtheria can lead to cardiac and neurologic complications, as well as death.
Immunization is the best prevention against diphtheria.
107
10.6 WHOOPING COUGH (PERTUSSIS)
What is whooping cough?
What is the history of whooping cough?
Can whooping cough be prevented with a vaccine?
What are whooping cough symptoms, signs, and stages?
How is whooping cough transmitted?
Can adults get whooping cough?
How is whooping cough diagnosed?
What is the treatment for whooping cough?
What are possible complications of whooping cough?
Where can people find more information about whooping cough (pertussis)?
10.6.1 What whooping cough is.
The disease is named for the characteristic sound produced when affected individuals
attempt to inhale; the whoop originates from the inflammation and swelling of the
laryngeal structures that vibrate when there is a rapid inflow of air during inspiration.
The first outbreaks of whooping cough were described in the 16th century.
The bacterium responsible for the infection, Bordetella pertussis, was not identified
until 1906.
Unimmunized or incompletely immunized young infants are particularly vulnerable to the
infection and its complications, which can include pneumonia and seizures.
10.6.2 Prevention with a vaccine.
Whooping cough commonly affects infants and young children but can be prevented
by immunization with pertussis vaccine.
Pertussis vaccine is most commonly given in combination with the vaccines for
diphtheria and tetanus.
(Pertussis is the "P" in the DTaP combination inoculation routinely given to children,
and the "p" in the Tdap vaccine administered to adolescents and adults.) Since
immunity from the pertussis vaccine wears off with time, many teenagers and adults
get whooping cough.
For maximum protection against pertussis, children need five DTaP shots.
The first three vaccinations are given at 2, 4, and 6 months of age. The fourth
vaccination is given between 15 and 18 months of age, and a fifth is given when a
child enters school, at 4-6 years of age.
Preteens going to the doctor for their regular checkup at 11 or 12 years of age should
get a dose of the Tdap booster, and adults who didn't get Tdap as a preteen or teen
should get one dose of Tdap.
The easiest way for adults to ensure immunity is to get the Tdap vaccine instead of
their next regular tetanus booster. (The Td shot is recommended every 10 years.)
To protect their infants, most pregnant women who were not previously vaccinated
with Tdap should get one dose of Tdap postpartum before leaving the hospital or
108
birthing center. Getting vaccinated with Tdap is especially important for mothers and
families with new infants as well as all people caring for newborns. Women planning
pregnancy may also choose to get vaccinated with Tdap prior to becoming pregnant.
In some cases, pregnant women may desire vaccination with the Tdap vaccine or may
be at risk for acquiring whooping cough.
The tetanus and diphtheria (Td) components of the vaccine are considered safe for
pregnant women. If the Tdap vaccine is given in pregnancy, the CDC recommends
that it be given in the second or third trimester.
Pregnant women should consult their health-care provider for a discussion their
individual situation regarding the pertussis vaccine.
10.6.3 Symptoms, signs, and stages of whooping cough
The first stage of whooping cough is known as the catarrhal stage. In the catarrhal stage,
which typically lasts from one to two weeks, an infected person has symptoms characteristic
of an upper respiratory infection, including:
runny nose,
sneezing,
low-grade fever,
Mild, occasional cough, similar to the common cold.
The cough gradually becomes more severe, and after one to two weeks, the second stage
begins. It is during the second stage (the paroxysmal stage) that the diagnosis of whooping
cough usually is suspected. The following characteristics describe the second stage:
There are bursts (paroxysms) of coughing, or numerous rapid coughs, apparently due
to difficulty expelling thick mucus from the airways in the lungs. Bursts of coughing
increase in frequency during the first one to two weeks, remain constant for two to
three weeks, and then gradually begin to decrease in frequency.
At the end of the bursts of rapid coughs, a long inspiratory effort (breathing in) is
usually accompanied by a characteristic high-pitched "whoop" sound.
During an attack, the individual may become cyanotic (turn blue) from lack of oxygen.
Children and young infants appear especially ill and distressed.
Vomiting (referred to by doctors as post-tussive vomiting) and exhaustion commonly
follow the episodes of coughing.
The person usually appears normal between episodes.
Paroxysmal attacks occur more frequently at night, with an average of 15-24 attacks
per 24 hours.
The paroxysmal stage usually lasts from one to six weeks but may persist for up to 10
weeks.
Infants under 6 months of age may not have the strength to have a whoop, but they do
have paroxysms of coughing.
The third stage of whooping cough is the recovery or convalescent stage. In the convalescent
stage, recovery is gradual. The cough becomes less paroxysmal and usually disappears over
two to three weeks; however, paroxysms often recur with subsequent respiratory infections
for many months.
109
10.6.4 Transmission
Whooping cough is highly contagious and is spread among people by direct contact
with fluids from the nose or mouth of infected people.
People contaminate their hands with respiratory secretions from an infected person and
then touch their own mouth or nose.
In addition, small bacteria-containing droplets of mucus from the nose or lungs enter
the air during coughing or sneezing. People can become infected by breathing in these
drops.
10.6. 5 Adults and whooping cough
Although whooping cough is considered to be an illness of childhood, adults may also
develop the disease.
The illness usually is milder in adults than in children, but the duration of the
paroxysmal cough is just as long as in children.
The characteristic whoop that occurs after paroxysmal bouts of coughing is recognized
in only 20%-40% of adults with whooping cough.
Because immunity from the pertussis vaccine decreases over time but does not
necessarily disappear, adults who do become infected may have retained a partial
degree of immunity against the infection that results in a milder illness.
Whooping cough in adults is more common than usually appreciated, accounting for
up to 7% of adult illnesses that cause coughing each year.
Infected adults are a reservoir (source) of infection for children, so it is particularly
important that all family members and caregivers of young infants be properly
vaccinated.
10.6.6 Diagnosis
When a patient has the typical symptoms of whooping cough, the diagnosis can be
made from the clinical history.
However, the disease and its symptoms, including its severity, can vary among
affected individuals.
In cases in which the diagnosis is not certain or a doctor wants to confirm the
diagnosis, laboratory tests can be carried out.
Culture of the bacterium Bordetella pertussis from nasal secretions can establish the
diagnosis.
Another test that has been used to successfully identify the bacterium and diagnose
whooping cough is the polymerase chain reaction (PCR) test that can identify genetic
material from the bacterium in nasal secretions.
10.6.7 Treatment for whooping cough.
Antibiotics directed against Bordetella pertussis can be effective in reducing the
severity of whooping cough when administered early in the course of the disease.
Antibiotic therapy can also help reduce the risk of transmission of the bacterium to
other household members as well as to others who may come into contact with an
infected person.
110
Unfortunately, most people with whooping cough are diagnosed later with the
condition in the second (paroxysmal) stage of the disease.
Treatment with antibiotics is recommended for anyone who has had the disease for
less than three to four weeks. Azithromycin (Zithromax), clarithromycin (Biaxin),
erythromycin (E-Mycin, Eryc, Ery-Tab, PCE, Pediazole, Ilosone), and trimethoprim
and sulfamethoxazole (Bactrim, Septra) are antibiotics which have been shown to be
effective in treating whooping cough.
It is unclear whether antibiotics have any benefit for people who have been ill with
whooping cough for longer than three to four weeks, although antibiotic therapy still is
often considered for this group.
There is no proven effective treatment for the paroxysms of coughing that accompany
whooping cough.
Antibiotics also are routinely administered to people who have had close contact with
an infected person, regardless of their vaccination status.
10.6.8 Complications of whooping cough
The most common complication and the cause of most whooping cough-related deaths
is secondary bacterial pneumonia.
Secondary bacterial pneumonia is bacterial pneumonia that follows another infection
of the lung, be it viral or bacterial. Secondary pneumonia is caused by a different virus
or bacterium than the original infection.
Young infants are at highest risk for whooping cough and also for its associated
complications, including secondary pneumonia.
Other possible complications of whooping cough, particularly in infants less than 6
months of age, include seizures, encephalopathy (abnormal function of the brain due
to decreased oxygen delivery to the brain caused by the episodes of coughing),
reactive airway disease (asthma), dehydration, hearing loss, and malnutrition.
Data indicate that secondary pneumonia occurs in about one out of every 20 infants
with whooping cough, and one out of 100 affected infants develop convulsions.
Whooping cough can cause serious illness and even death in young children
10.6.9 Conclusion
Whooping cough (pertussis) is an acute, highly contagious respiratory infection that is
caused by the bacterium Bordetella pertussis.
Whooping cough commonly affects infants and young children but can be prevented
by immunization with pertussis vaccine.
Adults may develop whooping cough as their immunity from childhood vaccines
wears off over time.
Clinical symptoms occur in three stages; the characteristic bursts of coughing are
observed in the second, or paroxysmal, stage.
Antibiotics can help reduce the severity of the disease when administered early in the
course of the disease.
Secondary bacterial pneumonia is the most common complication of whooping cough.
111
10.7 Summary
Poliomyelitis is a viral disease that can affect nerves and can lead to partial or full paralysis.
Poliomyelitis is a disease caused by infection with the poliovirus. The virus spreads by direct
person-to-person contact, by contact with infected mucus or phlegm from the nose or mouth,
or by contact with infected feces. The virus enters through the mouth and nose, multiplies in
the throat and intestinal tract, and then is absorbed and spread through the blood and lymph
system. Polio immunization (vaccine) effectively prevents poliomyelitis in most people
(immunization is over 90% effective).
Measles is best known for causing a rash in childhood, but measles can affect other parts of
the body and sometimes occurs in adults. Vaccination has significantly reduced the number of
cases in the United States, although isolated outbreaks continue to occur. The rubeola virus
causes "red measles," also known as "hard measles" or just "measles." Although most people
recover without problems, rubeola can lead to pneumonia or inflammation of the brain
(encephalitis). The rubella virus causes "German measles," also known as "three-day
measles." This is usually a milder disease than red measles. However, this virus can cause
significant birth defects if an infected pregnant woman passes the virus to her unborn child.
Symptoms occur in two phases with he early phase beginning with fever, A run-down feeling,
Cough, Red eyes (conjunctivitis), runny nose, loss of appetite. Second phase is characterized
with rash all over starting with the face, spreading to the trunk and then to the arms and legs.
There is no specific treatment or cure for measles. It can be prevented by immunization.
Diphtheria is an infectious disease caused by the bacterium Corynebacterium diphtheriae.
Diphtheria is primarily transmitted via airborne respiratory droplets or by direct contact with
secretions from infected people. The symptoms of diphtheria include sore throat, fever,
malaise, difficulty swallowing, and difficulty breathing. Diphtheria is treated with both
antitoxin and antibiotics. Diphtheria can lead to cardiac and neurologic complications, as well
as death. Immunization is the best prevention against diphtheria.
Whooping cough (pertussis) is an acute, highly contagious respiratory infection that is caused
by the bacterium Bordetella pertussis. Whooping cough commonly affects infants and young
children but can be prevented by immunization with pertussis vaccine. Adults may develop
whooping cough as their immunity from childhood vaccines wears off over time. Clinical
symptoms occur in three stages; the characteristic bursts of coughing are observed in the
second, or paroxysmal, stage. Antibiotics can help reduce the severity of the disease when
administered early in the course of the disease. Secondary bacterial pneumonia is the most
common complication of whooping cough.
10.8 Self-Test Questions
i)
ii)
What are poliomyelitis, measles, diphtheria and whooping cough?
Describe the causes, transmission, diagnosis, signs and symptoms, treatment,
control and prevention measures for poliomyelitis, measles, diphtheria and
whooping cough.
112
10.9 Further reading
United States. California Department of Public Health. "Whooping Cough Epidemic May Be
Worst in 50 Years." June 23, 2010. http://www.cdph.ca.gov/Pages/NR10-041.aspx.
United States. Centers for Disease Control and Prevention. "Pertussis Disease - Questions &
Answers (Whooping Cough)." June 2, 2010. http://www.cdc.gov/vaccines/vpdvac/pertussis/dis-faqs.htm.
Nath A, Berger JR. Poliomyelitis. In: Goldman L, Ausiello D, eds. Cecil Medicine. 23rd ed.
Philadelphia, Pa: Saunders Elsevier. 2007: chap 440.
Silver JK. Post-poliomyelitis syndrome. In: Frontera WR, Silver JK, Rizzo Jr TD, eds.
Essentials of Physical Medicine and Rehabilitation. 2nd ed. Philadelphia, Pa: Saunders
Elsevier; 2008: chap 137.
Mason WH. Measles. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, eds. Nelson
Textbook of Pediatrics. 18th ed. Philadelphia, Pa: Saunders Elsevier; 2007: chap 243.
113
LECTURE ELEVEN
SEXUALLY TRANSMITTED DISEASES (STDs)
11.1 Introduction
Welcome to the tenth lecture in our course. In this lecture we will cover sexually transmitted
diseases (STDs). The broader objective of this lecture is to enable you to describe sexually
transmitted diseases (STDs).
12.2 Objectives
By end of this lecture you should be able to:
i) Describe what sexually transmitted diseases are.
ii) Explain sexually transimitted diseases.
11.3 Overview of Sexually Transmitted Diseases (STDs)
Sexually transmitted diseases (STDs, venereal diseases) are among the most common
infectious diseases today. STDs are sometimes referred to as sexually transmitted
infections, since these conditions involve the transmission of an infectious organism
between sex partners.
More than 20 different STDs have been identified, and about 19 million men and
women are infected each year in the United States, according to the CDC (2010).
Depending on the disease, the infection can be spread through any type of sexual
activity involving the sex organs, the anus, or the mouth; an infection can also be
spread through contact with blood during sexual activity.
STDs are infrequently transmitted by any other type of contact (blood, body fluids or
tissue removed from an STD infected person and placed in contact with an uninfected
person); however, people that share unsterilized needles markedly increase the chance
to pass many diseases, including STD's (especially hepatitis B), to others.
Some diseases are not considered to be officially an STD (for example, hepatitis types
A, C, E) but are infrequently noted to be transferred during sexual activity.
Consequently, some authors include them as STD's, others do not.
Consequently, lists of STD's can vary, depending on whether the STD is usually
transmitted by sexual contact or only infrequently transmitted.
STDs affect men and women of all ages and backgrounds, including children.
STDs have become more common in recent years, partly because people are becoming
sexually active at a younger age, are having multiple partners, and do not use
preventive methods to lessen their chance of acquiring an STD.
People can pass STDs to sexual partners even if they themselves do not have any
symptoms.
114
Frequently, STDs can be present but cause no symptoms, especially in women (for
example, chlamydia, genital herpes or gonorrhea). This can also occur in some men.
Health problems and long-term consequences from STDs tend to be more severe for
women than for men. Some STDs can cause pelvic infections such as pelvic
inflammatory disease (PID), which may cause a tubo-ovarian abscess. The abscess, in
turn, may lead to scarring of the reproductive organs, which can result in an ectopic
pregnancy (a pregnancy outside the uterus), infertility or even death for a woman.
Human papillomavirus infection (HPV infection), an STD, is a known cause of cancer
of the cervix.
Many STDs can be passed from a mother to her baby before, during, or immediately
after birth.
Because the method of becoming infected is similar with all STDs, a person often
obtains more than one pathogenic organism at a time. For example, many people
(about 50%) are infected at a single sexual contact with both gonorrhea and
chlamydia.
11.3.1 Sexually Transmitted Diseases (STDs) Causes
Depending on the disease, STDs can be spread with any type of sexual activity. STDs are
most often caused by viruses and bacteria. The following is a list of the most common STDs,
their causes and other infections that may be transmitted on occasion by sexual activity, but
are frequently not considered primarily to be an STD by many investigators:
a) STDs caused by bacteria
Chancroid (Haemophilus ducreyi)
Chlamydia (Chlamydia trachomatis)
Gonorrhea (Neisseria gonorrhea)
Granuloma inguinale (Calymmatobacterium granulomatis)
Lymphogranuloma venereum (Chlamydia trachomatis)
Syphilis (Treponema pallidum)
b) STDs caused by viruses
Genital herpes (herpes simplex virus)
Genital warts (human papillomavirus virus [HPV])
Hepatitis B and D, and infrequently
HIV/AIDS (human immunodeficiency virus [HIV virus])
Molluscum contagiosum (poxvirus)
115
c) STD caused by protozoan
Trichomoniasis (Trichomonas vaginalis)
d) STD's caused by fungi
Jock itch (Tenia cruris)
Yeast infections (Candida albicans)
e) STD's caused by parasites
Pubic lice or crabs (Pediculosis pubis)
Scabies Sarcoptes scabiei
11.4 Sexually Transmitted Diseases (STDs) Symptoms
Common STDs have a variety of symptoms (if symptoms develop at all) and many different
complications, including death.
11.4.1 Symptoms of STDs caused by bacteria
Chancroid Symptoms
Common in developing countries.
Symptoms include painful ulcers on the genitals.
Can be confused with syphilis or herpes
Is treatable with antibiotics e.g. a single oral dose of 1 gm of azithromycin
(Zithromax) or a single injection of ceftriaxone (Rocephin). Alternative medications
are ciprofloxacin (Cipro) or erythromycin, 500 mg taken three times per day by mouth
for seven days.
Chlamydia symptoms
Most common of all STDs caused by bacteria.
Cause no symptoms in about 80% of women and 50% of men
When symptoms are present, commonly there is discharge from the vagina or the
penis, and burning or pain during urination.
Is transmitted through vaginal, oral, or anal sexual contact
Ectopic pregnancy and infertility for women are potential serious complications.
Is treatable with antibiotics e.g. azithromycin (Zithromax, Zmax) or doxycycline
(Vibramycin, Oracea, Adoxa, Atridox) and others
116
Gonorrhea symptoms
Gonorrhea is a bacterial infection caused by the organism Neisseria gonorrheae that is
transmitted by sexual contact. Gonorrhea is one of the oldest known sexually
transmitted diseases.
Contrary to popular belief, gonorrhea cannot be transmitted from toilet seats or door
handles. The bacterium that causes gonorrhea requires very specific conditions for
growth and reproduction. It cannot live outside the body for more than a few seconds
or minutes, nor can it live on the skin of the hands, arms, or legs.
It survives only on moist surfaces within the body and is found most commonly in the
vagina, and, more commonly, the cervix.
Discharge from the vagina or the penis
Over 50% of infected women have no symptoms, but they can still transmit the
disease to others.
Painful urination
Ectopic pregnancy, pelvic inflammatory disease (PID), infertility for women,
Fitzhugh-Curtis syndrome (perihepatitis) and death are potential serious
complications.
Is treatable with antibiotics e.g. penicillin, a single injection of ceftriaxone
intramuscularly or by 400mg of cefixime (Suprax) in a single oral dose and others.
Granuloma inguinale (donovanosis) symptoms
Symptoms are painless genital ulcers in the groin area.
Is treatable with antibiotics, usually for three or more weeks
Lymphogranuloma venereum
Symptoms are abscesses (buboes) in the groin, rectum or other areas; fistulas that
drain pus may occur and are treatable with antibiotics.
Syphilis
Symptoms are mild and often go undetected initially
Starts with a painless genital ulcer that goes away on its own
Rash, fever, headache, achy joints
Is treatable with antibiotics e.g. Long-acting penicillin injections, oral doxycycline or
tetracycline.
More serious complications associated with later stages of the disease if undetected
and untreated
117
11.4.2 Symptoms of STDs caused by viruses
i.
Genital herpes
Recurring outbreaks of blister-like sores on the genitals
Can be transmitted from a mother to her baby during birth
Reduction in frequency and severity of blister outbreaks with treatment but not
complete elimination of infection.
Can be transmitted by a partner who has herpes even if no blisters are present.
Although there is no known cure for herpes, there are treatments for the outbreaks.
There are oral medications, such as acyclovir (Zovirax), famciclovir (Famvir), or
valacyclovir (Valtrex) that prevent the virus from multiplying and even shorten the
length of the eruption.
ii.
Genital warts
Caused by a virus related to skin warts, human papillomavirus (HPV)
Small, painless bumps in the genital or anal areas (sometimes in large clusters that
look like cauliflower)
There is no cure or treatment that can eradicate HPV infection, so the only treatment is
to remove the lesions caused by the virus. Unfortunately, even removal of the warts
does not necessarily prevent the spread of the virus, and genital warts frequently recur.
None of the available treatments are ideal or clearly superior to others.
Various treatments available (for example, freezing or painting the warts with
medication), a 0.5% solution or gel of podofilox (podophyllotoxin), alternatively a
5% cream of imiquimod (a substance that stimulates the body's production of
cytokines, chemicals that direct and strengthen the immune response)
Vaccines are available against the most common types of HPV
iii.
Hepatitis
Hepatitis B and D are most often associated with sexual contact, hepatitis A, C, E are
less frequently transmitted by sexual contact.
Both may be transmitted via contact with blood; for hepatitis B, sexual transmission is
believed to be responsible for 30% of the cases worldwide.
The hepatitis B virus can cause both an initial (acute) and a chronic form of liver
inflammation. Only 50% of acute infections with the hepatitis B virus produce
symptoms. The initial phase of infection lasts a few weeks, and in most people (90%95%), the infection clears.
Acute infection can cause yellowish skin and eyes, fever, achy, tired (flu-like
symptoms).
118
Severe complications in some people, including cirrhosis and liver cancer may occur
in a small percent of individuals infected with HBV.
Treatments are available and remission is possible with some aggressive medications.
Immunizations are available to prevent hepatitis B.
iv.
HIV/AIDS
Spread primarily by sexual contact and from sharing needles
Can be transmitted at the time a person becomes infected with other STDs
No specific symptoms or physical signs confirm HIV infection.
The average time from infection to the development of symptoms related to
immunosuppression (decreased functioning of the immune system) is 10 years.
Fatigue, night sweats, chills, or fever lasting several weeks, headaches, and cough may
occur a few weeks after contracting the virus initially.
Serious complications of AIDS include unusual infections or cancers, weight loss,
intellectual deterioration (dementia), and death.
No current cure but medications are available to slow disease progression.
v.
Molluscum contagiosum
Small (2-5mm) raised areas (papules) on the skin
Contagious, usually by direct skin to skin contact
Self-limited over months to years; treated with some topical creams
Often cryotherapy (freezing) or surgical removal is performed
11.4.3 Symptoms of STDs caused by protozoan
i. Trichomonas
Frothy vaginal discharge with a strong odor
Treated with antibacterial/antiprotozoal medicines
11.4.4 Symptoms of STDs caused by fungi
i. Jock itch (genital itching or Tenia cruris) (not always an STD)
Itchy groin skin, sometimes has a reddish color
Is treated with topical antifungal medicines
119
ii. Yeast infection (Candidiasis) (not always an STD)
Cheese-like vaginal discharge or whitish exudates sometimes with a reddish hue to the
skin; it may occur around the foreskin of infected males; common symptoms are
itching and burning sensation of the vagina or penis.
Is treated with topical antifungal medicines in most cases
11.4.5 Symptoms of STDs caused by parasites
i) Pubic lice
Very tiny bugs that are found in pubic hair, sometimes referred to as "crabs"
Can be picked up from clothing or bedding
First noticed as itching in the pubic area
Are treatable with creams, anti-lice agents, and combing
ii.Scabies
Skin infestation caused by a tiny mite
Highly contagious
Intense itching is the primary symptom, which worsens at night
Spread primarily by sexual contact or from contact with skin, infested sheets, towels,
or furniture
Is treated with creams. Ivermectin (Stromectol) is a drug taken by mouth that has also
been successfully used to treat scabies.
11.4.6 Diagnosis
Some STDs can be diagnosed without any tests at all (for example, pubic lice).
Other STDs require a blood test or a sample of any unusual fluid (such as an abnormal
discharge from the vagina or the penis for gonorrhea or chlamydia) to be analyzed in a
lab to help establish a diagnosis.
Some tests are completed while a person waits; other tests require a few days before a
person may obtain the results (for example, syphilis).
11.5 Sexually Transmitted Diseases (STDs) Treatment
11.5.1 Self-Care at Home
Home treatment of STDs is not recommended because prescription medications are
usually necessary.
120
The treatment of an STD varies depending on the type of STD. Drugs can be taken
either by:
i. Mouth
ii. Injection or
iii. Apply creams or special solutions on the skin.
Often, reexamination by a doctor is necessary after the treatment to confirm that the
STD is completely gone.
Some STDs, such as genital herpes and HIV (which leads to AIDS), cannot be cured,
only controlled with medication.
The sooner a person seeks treatment and warns sexual partners about the disease, the
less likely the disease will do permanent damage, be spread to others, or be passed to a
baby.
11.6 Prevention
The best way to prevent STDs is to avoid sexual contact with others. If people decide to
become sexually active, they can reduce the risk of developing an STD in these ways:
Practice abstinence (refrain from sex entirely)
Be faithful in a monogamous relationship (both sexual partners are each other's only
sexual partner).
Delay having sexual relations as long as possible. The younger people are when they
become sexually active, the higher the lifetime risk for contracting an STD. The risk
also increases with the number of sexual partners.
Correctly and consistently use a male latex condom. In addition, condoms are only
about 90% effective in preventing STDs
The spermicide nonoxynol-9, once thought to protect against STDs as well as to
prevent pregnancy, has been proven to be ineffective for disease prevention. Do not
rely on it.
Have regular medical checkups even if you do not have symptoms of an STD.
Learn the symptoms of STDs.
Avoid douching because it removes some of the natural protection in the vagina.
Vaccines against HPV and hepatitis B are available and effective.
11.7 Summary
Sexually transmitted diseases (STDs, venereal diseases) are among the most common
infectious diseases today. STDs are sometimes referred to as sexually transmitted infections,
since these conditions involve the transmission of an infectious organism between sex
partners.
121
Depending on the disease, the infection can be spread through any type of sexual activity
involving the sex organs, the anus, or the mouth; an infection can also be spread through
contact with blood during sexual activity.
STDs are infrequently transmitted by any other type of contact (blood, body fluids or tissue
removed from an STD infected person and placed in contact with an uninfected person);
however, people that share unsterilized needles markedly increase the chance to pass many
diseases, including STD's (especially hepatitis B), to others.
STDs caused by bacteria include: Chancroid (Haemophilus ducreyi), Chlamydia (Chlamydia
trachomatis), Gonorrhea (Neisseria gonorrhea), Granuloma inguinale (Calymmatobacterium
granulomatis), Lymphogranuloma venereum (Chlamydia trachomatis), Syphilis (Treponema
pallidum). STDs caused by viruses include: Genital herpes (herpes simplex virus), Genital
warts (human papillomavirus virus [HPV]), Hepatitis B and D, and infrequently, HIV/AIDS
(human immunodeficiency virus [HIV virus]), Molluscum contagiosum (poxvirus). STD
caused by protozoan include: Trichomoniasis (Trichomonas vaginalis). STD's caused by fungi
include: Jock itch (Tenia cruris), Yeast infections (Candida albicans). STD's caused by
parasites include: Pubic lice or crabs (Pediculosis pubis), Scabies Sarcoptes scabiei.
Sexually transmitted infections can be prevented by use of various ways as discussed above.
11.8 Self-Test Questions
i) Describe what sexually transmitted diseases are.
ii) Explain the causes, transmission, signs and symptoms prevention and control
of bacterial, fungal, parasitic, and viral sexually transmitted diseases you
know.
11.9 Further reading
Fauci, Anthony S., et al. Harrison's Principles of Internal Medicine. 17th ed. United States:
McGraw-Hill Professional, 2008.
122
LECTURE TWELVE
EMERGING AND RE-EMERGING DISEASES
12.1 Introduction
Welcome to the eleventh lecture in our course. In this lecture we will cover emerging and reemerging diseases. The broader objective of this lecture is to enable you to describe emerging
and re-emerging diseases.
12.2 Objectives
By end of this lecture you should be able to:
i) Describe what emerging and re-emerging diseases are.
ii) Describe emerging and re-emerging diseases.
iii) Explain factors contributing to the emerging and re-emerging diseases.
12.3 Emerging and Re-Emerging Diseases
Emerging and re-emerging infection pose serious public health threat to most countries of the
word. In the past decade, epidemics of diseases including Influanza, dysentery, chorela
menengits, yellow fever and Ebola virus have resulted in significant morbidity and mortality.
As diseases like smallpox are eradicated some new diseases emerge and take their place.
12.3.1 Emerging diseases
These are diseases which had never been recognised before. They include: AIDS/HIV, SARS
(severe acute respiratory syndrome), Nipah virus encephalitis, and variant Creutzfeld-Jakob
diseases (VCJD).
In sub-Saharan Africa, two groups of emerging diseases that are a threat include viral
haemorrhagic fevers (Ebola, Lassa and Marbug) and acute respiratory illnesses (SARS,
Avianinfluenza).
12.3.2 Re-emerging, resurging diseases.
They are those that have been around for decades or centuries, but have come back in a
different form or a different form or a different location. Examples are West Nile virus,
monkey pox in USA, dengue fever in South America and Caribbean, and malaria and
Tuberculosis as global pandemics
12.3.2 Factors which have contributed to emerging and re-emerging diseases.
They include:
- economic development and land use
- human demograph and behavior
123
- internal traveland trade
- breakdown in the health infrastructure
- enroachment of human civilization on the environment
- changes in social structure and human behavious
12.4 HIV/AIDS
HIV/AIDS was first described in the scientific literature in June 1981.
Because the AIDS pandemic is now more than 28 years old, it will soon be considered
one of the fundamental matrix diseases.
However, in 1981, it was truly an emerging disease. Nearly 40 million people
throughout the world are currently infected with HIV, two thirds of whom live in subSaharan Africa.
When HIV/AIDS first emerged, there were many misunderstandings. Initially we
measured the time from infection to disease manifestations in months or very few
years.
It now appears that without therapy there is a median 10-year time period before
advanced HIV disease or AIDS develops.
So those patients who were first identified in 1981 had likely been infected with HIV
for many years before developing symptoms of the advanced disease.
Despite the current concentration of disease burden in sub-Saharan Africa, Asia likely
will be the next epicenter of this disease. Currently about five million people in India
are infected with HIV, but that is less than 1 percent of the adult population. In
Botswana, 37 percent of the adult population is infected, but the population is only 1.6
million.
If the prevalence rate in India, which has a total population of more than a billion,
were to approach what we are seeing in Africa, the impact would be enormous.
Of the estimated 6.5 million low- and middle-income people in other countries who
need treatment, most of whom live in sub-Saharan Africa, only 15 percent are
receiving antiretroviral drugs. However, there is a lot of progress.
Unless we slow the rate of new HIV infections, continued treatment of significant
numbers of AIDS patients throughout the world will not be feasible, neither
logistically nor financially. This brings us to the issue of HIV prevention.
We often lose sight of the fact that HIV/AIDS is a totally preventable disease. There
are multiple modalities of prevention, and some of them work very well. The various
strategies, including
-
Education,
Behavior modification,
Treatment of drug users,
Distribution of condoms,
Clean syringes,
Needle exchange programs,
Topical microbicides,
Antiretroviral therapy, and
Abstinence can prevent HIV/AIDS,
124
All of these approaches must be pursued concomitantly.
In a perfect world, we would not need an HIV/AIDS vaccine, but we do not live in a
perfect world and people continue to put themselves and others at risk. Hence, a
vaccine is needed. The scientific challenge of developing an effective HIV vaccine is
formidable.
For every other major infectious disease—even the great killers and maimers, such as
smallpox, polio, and measles—the body handles the invading microbes the vast
majority of the time without a vaccine. Historically, only 25 to 30 percent of people
infected with the smallpox virus die; 70 to 75 percent of those infected recover
spontaneously.
Similarly, more than 98 percent of people infected with poliovirus never experience
serious complications. The reason is that the body has the capability of ultimately
mounting an effective immune response, generally an antibody response, to eliminate
the microbe.
No such luck with HIV. Of the more than 60 million people who have been infected
with HIV since the beginning of the epidemic, there is not a single well-documented
case of someone who has completely cleared the virus after having had an established
infection. Lymphocyte cultures from people who clinically are doing very well still
yield the virus. Even for people on drug therapy for eight to nine years with
undetectable viral loads, the virus can be cultured from their lymphocytes.
12.5 Malaria and Tuberculosis
Malaria is one of those diseases that most people in the developed world just do not
think about. Yet more than one million people with malaria die each year. Every 30
seconds, a child dies of malaria.
Over the past few years we have made considerable progress in malaria research.
Scientists now have completed the genomic sequence of the most virulent malariacausing parasite, Plasmodium falciparum, as well as that of Anopheles gambiae, one
of the important mosquito species that carries the parasite. With the human genome
sequence also available, we have the genomes of the host, the vector, and the microbe
completely sequenced.
This information can now be helpful in the design of effective drugs and vaccines as
well as in other areas of malaria control. More recently, a trial of a malaria vaccine
conducted among children in Mozambique showed 30 percent efficacy in preventing
infection and nearly 60 percent efficacy in preventing severe disease. Although the
vaccine is not as efficacious as most commonly used childhood vaccines, it has the
potential for saving millions of lives.
Tuberculosis is another major killer, causing the deaths of about two million people
each year. About one third of the world’s population is infected with the
mycobacterium that causes tuberculosis, and almost four million have the active
disease at any time, including the 300,000 new cases of multiple-drug-resistant
tuberculosis that develop each year.
Tuberculosis is especially prevalent among patients with HIV/AIDS: about 46 percent
of people in the developing world with HIV are co-infected with tuberculosis, and 13
percent of the deaths among HIV-infected individuals are from disseminated
tuberculosis.
125
12.6 Influenza
Influenza, as common as it is, is a greatly misunderstood disease. Each year we
confront seasonal, or interpandemic, influenza.
Seasonal influenza kills about 250,000 to 300,000 people each year throughout the
world. A pandemic occurs through exposure to a microbe for which there is no
baseline immunity in the population.
The worst influenza pandemic in history occurred in 1918–1919. There were 40
million deaths throughout the world.
Unlike the seasonal flu that typically kills the elderly, the 1918 pandemic killed young
people as well. Even though they were fundamentally healthy, 20-, 30-, and 40-yearold people were dying because they had no background immunity to the virus, and the
virus was particularly virulent.
In 1918, H1N1 first appeared; in 1957 H2N2 emerged, and in 1968, we first saw
H3N2.
126
Over the past few years, many more influenza strains have emerged with the
capability of infecting humans.
The H5N1 strain likely evolved from a few flocks of chickens in Hong Kong (where it
was first noticed in 1996 and infected a small number of humans in 1997) to the
situation today where it has infected numerous flocks, as well as wild birds throughout
Southeast Asia.
12.7 SARS
This is severe acute respiratory syndrome (SARS).
The morbidity and mortality associated with the SARS outbreak were not as great as
what we observe every year with influenza.
SARS first appeared in Guangdong Province in China. It was not reported to
authorities until it emerged in Hong Kong, when an index case, who traveled from
Guangdong to Hong Kong, stayed at the Metropole hotel and infected at least 14
people.
Those individuals did some traveling throughout the world. Within months we had an
epidemic that temporarily transfixed the world and did extraordinary economic
damage in Canada, China, and Hong Kong, and other countries. There were 8,098
reported cases and 774 deaths.33
12.8 West Nile Virus
West Nile virus is transmitted to human by a mosquitobite. The virus can cause
encephatis or meningitis. The virus was discovered in 1937in the west Nile district of
Uganda.
Whereas SARS is an emerging infection, West Nile virus is a re-emerging infection. It
has existed in Africa and the Middle East for decades, if not centuries.
In 1999, it landed in Queens, New York, via an unknown route. The infection then
proceeded to move across the country, with different hot spots each year.
In 1999, the hotspot was Long Island and New York City. In the year 2000, it spread a
little bit, and many people were not terribly worried. However, all the makings for an
epidemic were in place: the right mosquito, the right microbe, and suitable hosts.
In 2001, the hotspots expanded throughout New York State and to Connecticut,
Maryland, and Florida. In 2002, hotspots appeared in the Midwest, and farther west in
2003.
By 2004, the epidemic had crossed the Rocky Mountains and emerged in California,
Arizona, and Colorado. In 2004, 2,470 cases and 88 deaths were reported.
West Nile is likely to follow the path of other infectious encephalopathies such as St.
Louis encephalitis and eastern equine encephalitis, becoming part of the background
matrix of infectious diseases.
Signs and symptoms of West Nile virus infection range from no symptoms at all to
rapidly fatal brain infection. In areas where the virus is common, people are likely to
show no symptoms of the infection or have a mild flu-like illness rather than a severe
brain infection. There is abrupt onset of fever, muscle aches and headaches
The research enterprise also has moved rapidly to respond to West Nile virus. In
particular, scientists have created a chimeric vaccine against West Nile virus. Genes
coding for the immunodominant antigens of the West Nile virus (a flavivirus) were
127
spliced into the genome of an attenuated yellow fever virus (another flavivirus)
vaccine to yield a chimeric yellow fever/West Nile virus vaccine.
This vaccine is now in clinical trials with promising early results. A similar chimeric
vaccine has also been created with an attenuated dengue virus vector.
Other West Nile vaccine approaches also are being pursued, including a DNA vaccine
and recombinant subunit vaccines, as well as new approaches to therapy.
12.9 Marburg Virus
This virus, related to Ebola virus, was first detected in 1967 in Marburg, Germany,
when people working with monkeys from Uganda became infected, resulting in seven
deaths.
Throughout the years in Kenya, South Africa, the Democratic Republic of the Congo,
and most recently, Angola, the virus has re-emerged. Fortunately, Marburg and Ebola
outbreaks tend to appear in localized regions and have not triggered epidemics
throughout the world.
Unlike influenza, which spreads even when people are relatively asymptomatic, Ebola
and Marburg are generally transmitted from people who are deathly ill.
The people at greatest risk of contracting disease are family members, physicians, and
nurses in hospitals, undertakers, and other people who come in close contact with
infected individuals.
Unless the virus is used as an agent of bioterrorism, it is unlikely that the world will
experience an epidemic of Ebola or Marburg, merely because of the somewhat
restricted manner of its transmission.
12.10 Rift valley fever.
This is a zoonotic disease.
It was first isolated in Rift valley province in Kenya in 1931 among sheep on a farm
where it occurred as a wavw of unexplained abortions in livestock.
Since then, outbreaks have occurred in Kenya most notable being during El Nino rains
in 1997/98 and floods of 2006/07. It has been reported in Saudi Arabia, Egypt,
Somalia and Yemen.
It primarily spread among animals by the bite of infected mosquitoes.
The disease may be severe in many domesticated animals including sheep,camel,
goats,and cattle, with an abortion rate of 100% among sheep.
During epizootics people may become infected either by infected mosquitoes or
through contact with blood, other body fluids or tissues of infected animals. This
contacts may occur during care of the animals, slaughtering or drinking raw milk.
Incubation period varies from 2 to 6 days
Symptoms and signs include: flu-like illness with sudden onset of fever, headache,
backache, muscle pain, vomiting and stiffening of the neck and photophobia lasting
for about 4-7 days. It may present severe symptomsof meningo-encephelitis,
conjunctivitis and heamorrhagic fever. Death in patients with meningo-encephelitis is
common.
Prevention is mainly through sustained animal vaccination with inactivatedvaccine
currently being used experimentally to protect veterinary and laboratory workers.
Gloves and other protective gear should be worn and appropriate care taken when
handling sick animals.
128
12.11 Bioterrorism
One of those days in history that we will never forget is September 11, 2001. Barely
had the dust settled on Ground Zero in New York City and the Pentagon when an
unknown bioterrorist sent anthrax spores through the mail, resulting in 22 anthrax
cases and five deaths.
A large infusion of resources was invested in bioterrorism research, and the issue of
how such research should be conducted was the subject of much debate.
Such research should be done openly and transparently by researchers who are
studying emerging and re-emerging diseases anyway.
12.12 Chikungunya.
The term is derived from the word Kungunyala in Makonde language of south-eastern
Tanzania and northern Mozambique, which means “that which bends up”. It refers to
the stooped posture many patients develop as a result of painful and debilitating
arthritis commonly associated with the disease.
Chikungunya fever is a viral disease transmitted to humans by the bite of infected
mosquitoes.
The virus is Alphavirus indigenious to tropical Africa and Asia.
It was first recognized in Tanzania in 1953 and has since been identified and cited as
the cause of numerous epidemics throughout Africa and many areas of Asia. It is
considered a re-emerging disease of considerable.
The vectors of Chikungunya are mainly
Signs and symptoms include: fever, headache, fatigue, nausea, vomiting, muscle pain,
rash and joint pain. Bleeding from the nose or gum is also possible in some cases.
12.12 Self-Test Questions
i) What are emerging and re-emerging diseases?
ii) Describe various emerging and re-emerging diseases.
iii) Explain factors contributing to the emerging and re-emerging diseases.
129
LECTURE THIRTEEN
GENDER ASPECTS OF COMMUNICABLE DISEASES
13.1 Introduction
Welcome to the thirteenth lecture in our course. In this lecture we will cover gender aspects of
communicable diseases. The broader objective of this lecture is to enable you to describe
aspects of communicable diseases in relation to gender.
13.2 Objectives
By end of this lecture you should be able to:
i) Describe various gender aspects of communicable diseases.
ii) Explain the factors which influence gender inequalities in
communicable diseases.
13.3 Gender inequalities in tuberculosis (TB)
There are higher incidences of TB in young and early middle-aged women in both
industrialized areas and poorer countries. This raises the question of whether:
-
Underdetection of TB
Accessing health care (poorer countries).
13.4 Gender and HIV/AIDS
Research over the past decade has revealed that gender roles and power relations directly and
indirectly influence a person's risk of and vulnerability to HIV infection.
Gender is also a factor in determining the level and quality of care, treatment and support that
HIV-positive men and women receive.
The anatomical make up and physiological structure of women put them at a higher risk of
infection than men.
13.5 Schistosomiasis, leishmaniasis, onchocerciasis, lymphatic filariasis, Chagas disease,
African trypanosomiasis and leprosy
These are diseases which continue to inflict considerable suffering and death amongst
poor populations in developing countries.
As with almost all infectious diseases, prevalence rates between and within the sexes
vary according to the roles and responsibilities assigned to men and women in their
respective biophysical environments.
130
Exposure to a given disease may be common for both sexes but are affected
significantly by age, race, income level, and occupational category.
On the other hand, even when men and women in the same cohort group share similar
economic and social situations, their physiological structures may respond to the same
hazard in different ways.
How the sexes respond differently to similar risks warrants much more research.
13.5.1 Schistosomiasis
Schistosomiasis results from infection with schistome trematode worms transmitted by
one of several species of water snail.
Where gender assigns to women the role of washing clothes and fetching water their
contact with contaminated water will place them at greater risk than the men in their
communities.
Where men are more exposed, as through fishing, they will be at greater risk than
women.
Regardless of exposure, the consequences for men and women are different.
In women, schistosomiasis has been associated with infertility, abortion, pre-term
delivery and life-threatening conditions such as extra-uterine pregnancy.
Female genital schistosomiasis (FGS) has been associated with increased vulnerability
to HIV infection and studies are currently underway to verify this association.
Because the symptoms of urinary and genital schistosomiasis may sometimes be
confused with sexually transmitted diseases, there may be stigma associated with
infection such that women either delay going to the health service or they consult
traditional healers.
13.5.2 Leishmaniasis, onchocerciasis and lymphatic filariasis
Leishmaniasis, onchocerciasis and lymphatic filariasis are all parasitically- induced
diseases which also have different consequences for men and women.
For example, hydrocoele, the genital manifestations of lymphatic filariasis in men,
presents as a chronic swelling of the scrotum and affects about 27 million men.
The experience of the disease is significantly influenced by gender. Detection is
difficult, as hydrocoele is associated generally with sexual disability and lowers the
productivity and wage-earning capacity of those afflicted.
Likewise, the experience of leishmaniasis, onchocerciasis and leprosy is gendered as
all are disfiguring infections, affecting the responses that the affected man or woman
elicits from others with whom he/she comes into contact. Women may depend more
on their physical appearance to enhance their prospects for marriage and sustaining a
partnership with a male.
Studies in a number of different regions of the world indicate that both cutaneous and
visceral leishmaniasis (as with tuberculosis) are more likely to be detected in men than
women through passive case finding, through active case finding females are detected
with much greater frequency.
In the case of cutaneous leishmaniasis, the disease is not permanently incapacitating
and women responsible for home management, are more likely to consult a traditional
healer than men, who are more likely to be detected and treated in the health centers.
Onchocerciasis, or river blindness, infects 18 million people mostly in West Africa.
131
This disease provides an interesting example of how sex and gender interact to elicit a
different response from the health system depending on whether men or women are
affected.
In the case of onchocerciasis, women often were excluded from receiving the
treatment drug ivermectin on the basis of their reproductive function, when such
exclusion was not warranted. The Special Programme for Research and Training in
Tropical Disease (TDR) proved that the drug ivermectin could prevent the disease
with one tablet a year.
However, Merck & Co., the pharmaceutical company that developed ivermectin,
recommended that pregnant women and mothers breast-feeding newborns should be
excluded from treatment until they were no longer pregnant or breast-feeding, at
which time they should receive treatment.
In endemic areas, this represents up to 30% of women. A TDR-sponsored study found
that women who had been excluded for these reasons did not seek treatment once they
became eligible for it.
Many cited the high cost of transportation, but the majority noted that they did not
know where to get treatment and preferred to wait for the mass campaign. By the time
such a yearly campaign was undertaken they were again pregnant or breast-feeding.
Repeated exclusion from treatment allows these women to become a reservoir for
disease transmission.
13.6 Summery
This section has attempted to provide an overview of the impact of gender on
communicable diseases.
There are many diseases that have not been included in this discussion that have
significant gendered implications in the way they are contracted and experienced.
Trachoma, for example, is more prevalent in women than in men and has an important
gender dimension.
Diarrhoeal disease, one of the major killers of children in the developing world, has
generated a considerable amount of social science research. Such research has
focussed on detecting the degree to which women’s productive and reproductive roles
are precipitating or protective factors for their offspring’s diarrhoea, and to explore
how women can be called upon again to manage these diseases.
The main objective of this review has been to stimulate the reader to begin to apply a
gender perspective to examine the health-illness-care process related to infectious
diseases.
13.7 Self-Test Questions
i)
ii)
Describe various gender aspects of communicable diseases.
Explain the factors which influence gender inequalities
communicable diseases.
132
in
REFERENCES
Abiose A. Onchocercal eye disease and the impact of Mectizan treatment. Ann Trop Med
Parasitol. 1998;92(Suppl 1):S11–22.
Albanese G, Venturi C, Galbiati G (January 2001). "Treatment of larva migrans cutanea
(creeping eruption): a comparison between albendazole and traditional therapy". Int. J.
Dermatol. 40 (1): 67–71. doi:10.1046/j.1365-4362.2001.01103.x. PMID 11277961.
http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=00119059&date=2001&volume=40&issue=1&spage=67.
Albiez EJ, Büttner DW, Duke BO. Diagnosis and extirpation of nodules in human
onchocerciasis. Trop Med Parasitol. 1988;39(Suppl 4):331–46.
Alonso, P.L., J. Sacaral, J.J. Aponte, et al. 2004. Efficacy of the RTS, S/AS02A Vaccine
against Plasmodium falciparum Infection and Disease in Young African Children:
Randomized Controlled Trial. Lancet 364:1411–20.
Amebiasis (amoebic dysentery). New York State Department of Health website. Available at:
http://www.health.state.ny.us/diseases/communicable/amebi...s/fact_sheet.htm.
Accessed
December 3, 2006.
Arthur Allen. Hepatitis C sweeps Egypt, Salon.com, 10 March 2000
Baird JK, Mistrey M, Pimsler M, Connor DH (March 1986). "Fatal human ascariasis
following secondary massive infection". Am. J. Trop. Med. Hyg. 35 (2): 314–8. PMID
3953945. http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=3953945.
Ball, P. A. J.; Gilles, Herbert Michael (1991). Hookworm infections. Human Parasitic
Diseases. 4. Amsterdam: Elsevier. pp. 38–39. ISBN 0-444-81243-1.
Bank. (2003) School deworming at a glance. Washington (D.C.): World Bank. 4 p.
Brieger WR, Awedoba AK, Eneanya CI, et al. The effects of ivermectin on onchocercal skin
disease and severe itching: results of a multicentre trial. Trop Med Int Health. 1998;3:951–61.
Bethony, Brooker, Albonico, Geiger, Loukas, Diemert and Hotez, (2006) Soil-transmitted
helminth infections: ascariasis, trichuriasis, and hookworm, Lancet 367:1521–1532.
Bethony, Jeffrey M., Gary Simon, David J. Diemert, et al. “Randomized, placebo-controlled,
double-blind trial of the Na-ASP-2 hookworm vaccine in unexposed adults.” Vaccine 26
(2008): 2408-2417.
Berman, J. D. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments
in the last 10 years. Clin Infect Dis 1997;24:684-703.
Bolbol AS (1992). "Risk of contamination of human and agricultural environment with
parasites through reuse of treated municipal wastewater in Riyadh, Saudi Arabia". J Hyg
Epidemiol Microbiol Immunol 36 (4): 330–7. PMID 1300348.
133
Brody, Jane E. “Babies know: a little dirt is good for you.” New York Times 26 January 2009.
Brooker, Clements, Bundy (2007) Global epidemiology, ecology and control of soiltransmitted helminth infections. Adv Parasitol 62:221-261
Brooker S, Hotez PJ, Bundy DAP (2008) Hookworm-Related Anaemia among Pregnant
Women: A Systematic Review. PLoS Negl Trop Dis 2(9): e291.
Brooker, Simon, Willis Akhwale, Rachel Pullan, et al. “Epidemiology of plasmodiumhelminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects
for combining control.” American Jounral of Tropical Medicine Hygiene 77 (2007): 88-98.
Brooker, Simon, Archie CA Clements, Peter J. Hotez, et al. “The co-distribution of
Plasmodium falciparum and hookworm among African schoolchildren.” Malaria Journal 5
(2006): 99-107.
Burnham G. Onchocerciasis. Lancet. 1998;351:1341–6.
"CDC Factsheet: Hookworm", accessed September 29, 2008
Centers for Disease Control and Prevention. 1981. Pneumocystis Pneumonia—Los Angeles.
Morbidity and Mortality Weekly Report 30:250–52.
Centers for Disease Control and Prevention. 1981. Kaposi’s Sarcoma and Pneumocystis
Pneumonia among Homosexual Men—New York City and California. Morbidity and
Mortality Weekly Report 30:305–8.
Central Intelligence Agency. 2005. The World Factbook 2005. http://www.cia.gov/cia/
publications/factbook/index.html. Accessed on September 8, 2005.
Charnock, Anne (1980) Taking Bilharziasis out of the irrigation equation. New Civil
Engineer, 7 August. 1980 Bilharzia caused by poor civil engineering design due to ignorance
of cause and prevention
Centers for Disease Control and Prevention. 2004. Cases of HIV Infection and AIDS in the
United
States,
2003:
HIV/AIDS
Surveillance
Report.
http://www.cdc.gov/
hiv/stats/2003SurveillanceReport.pdf.
Centers for Disease Control and Prevention. West Nile Virus. http://www.cdc.gov/ncidod/
dvbid/westnile/index.htm. Accessed on September 8, 2005.
Crompton DW (June 1999). "How much human helminthiasis is there in the world?". J.
Parasitol. 85 (3): 397–403. doi:10.2307/3285768. PMID 10386428.
Croese J, O'neil J, Masson J, et al. (January 2006). "A proof of concept study establishing
Necator americanus in Crohn's patients and reservoir donors". Gut 55 (1): 136–7.
doi:10.1136/gut.2005.079129. PMID 16344586.
http://gut.bmj.com/cgi/pmidlookup?view=long&pmid=16344586
134
Cockburn, T A (1963). The evolution and eradication of infectious diseases, Baltimore, MD:
Johns Hopkins Press
Cockburn, T.A. 1963. The Evolution and Eradication of Infectious Diseases. Baltimore, MD:
Johns Hopkins Press.
Cooper, P.J. “Intestinal worms and human allergy.” Parasite Immunology 26 (2005): 455-467.
Correale J, Farez M (February 2007). "Association between parasite infection and immune
responses in multiple sclerosis". Ann. Neurol. 61 (2): 97–108. doi:10.1002/ana.21067. PMID
17230481.
Daily Mail. The bloodsucking worm that fights allergies from inside your tummy September
14, 2007.
David and Williami. Markell and Voge's Medical Parasitology. Philadelphia: Saunders, 2006
Desjeux, P. Leishmaniasis: public health aspects and control. Clin Dermatol 1996;14:417-23.
Deworm the World at Clinton Global Initiative 2008 Annual Meeting: up to 10 million
children to benefit from deworming! Press Release. Deworm the World, 2008.
Diemert, David J., Jeffrey M. Bethony, and Peter J. Hotez. “Hookworm Vaccines.” Vaccines
46 (2008): 282-288.
Drugs for parasitic infections. Med Lett Drugs Ther. 2007;5(Suppl):e1–15.
Embil JA, Pereira LH, White FM, Garner JB, Manuel FR (July 1984). "Prevalence of Ascaris
lumbricoides infection in a small Nova Scotian community". Am. J. Trop. Med. Hyg. 33 (4):
595–8. PMID 6476203. http://www.ajtmh.org/cgi/pmidlookup?view=long&pmid=6476203
Fauci, A.S. 2001. Infectious Diseases: Considerations for the 21st Century. Clinical Infectious
Diseases 32:675–85.
Fauci, Anthony S., et al. Harrison's Principles of Internal Medicine. 17th ed. United States:
McGraw-Hill Professional, 2008.
Fauci, A.S. 2003. HIV and AIDS: 20 Years of Science. Nature Medicine 9:839–43.
Fleming et al. (2006) Synergistic associations between hookworm and other helminth species
in a rural community in Brazil Tropical Medicine and International Health 11(1):56-64
Folkerts G, G. Walzl, P.J. Openshaw. “Do common childhood infections teach the immune
system not to be allergic?” Immunology Today 21 (2000): 118-120.
Fraser, C.M., J.A. Eisen, K.E. Nelson, I.T. Paulsen, and S.L. Salzberg. 2002. The Value of
Complete Microbial Genome Sequencing (You Get What You Pay For). Journal of
Bacteriology 184:6403–05.
135
Gasser, Cantacessi, Campbell (2009) Improved molecular diagnostic tools for human
hookworms. Expert Rev Mol Diagn 9(1):17-21
Gasser, Robin B., Cinzia Catacessi, and Bronwyn E. Campbell. “Improved molecular
diagnostic tools for human hookworms.” Expert Reviews 9 (2009): 17-21.
Gandhi et al. (2001) Epidemiology of Necator Americanus Hookworm Infections in
Xiulongkan Village, Hainan Province China: High Prevalence and Intensity Among MiddleAged and Elderly Residents. Journal of Parasitology. 87(4):739-743
Gardner, M.J., N. Hall, E. Fung, et al. 2002. Genome Sequence of the Human Malaria
Parasite Plasmodium falciparum. Nature 419:498–511.
Garrett, L. 1994. The Coming Plague. New York: Farrar, Straus and Giroux.
Global network for neglected tropical diseases receives $34 million from Gates Foundation:
IDB leads campaign to greatly reduce the burden of most neglected diseases by 2020 in Latin
America and the Caribbean.” Press Release. Global Network for Neglected Tropical Diseases.
30 January 2009.
Gyorkos, Larocque, Casapia, Gotuzzo. (2006)Lack of Risk Adverse Birth Outcomes After
Deworming in Pregnant Women The Pediatric Infectious Disease Journal 25(9): 791-794
Gyorkos, Larocque, Casapia, Gotuzzo. (2006)Lack of Risk Adverse Birth Outcomes After
Deworming in Pregnant Women The Pediatric Infectious Disease Journal 25(9): 791-794
Habbari K, Tifnouti A, Bitton G, Mandil A (September 1999). "Helminthic infections
associated with the use of raw wastewater for agricultural purposes in Beni Mellal, Morocco".
East. Mediterr. Health J. 5 (5): 912–21. PMID 10983530.
Haslett, E, et al., (2002). Davidson’s principles and practice of medicine (19th ed), Edinburgh:
Churchill Livingstone
Hawdon, Hotez, (1996) Hookworm: developmental biology of the infectious process Current
Opinion in Genetics and Development 6(5):618-623
Hawker, J, et al.,(2005). Communicable diseases control handbook, Massachusetts: Blackwell
Publishing Inc
Herwaldt, B. L. Leishmaniasis. Lancet 1999;354:1191-9.
Herwaldt, B. L., Stokes, S. L., Juranek, D. D. American cutaneous leishmaniasis in U.S.
travelers. Ann Intern Med 1993;118:779-84.
Heymann, D (ed) (2004). Control of communicable diseases manual, Washington, DC:
American Public Health Association
Hirschberg, R., J. La Montagne, and A.S. Fauci. 2004. Biomedical Research—An Integral
Component of National Security. New England Journal of Medicine. 350:2119–21.
136
Hoerauf H, Mand S, Adjei O, et al. Depletion of Wolbachia endobacteria in Onchocerca
volvulus by doxycycline and microfilaridermia after ivermectin treatment. Lancet. 2001;
357:1415–6.
Hookworms." The Center for Food Security and Public Health. May 2005. Iowa State
University.
Holt, R., G.M. Subramanian, A. Halpern, et al. 2002. The Genome Sequence of the Malaria
Mosquito Anopheles gambiae. Science 298:129–49.
Horák P (1992). "Helminth eggs in the sludge from three sewage treatment plants in
Czechoslovakia". Folia Parasitol. 39 (2): 153–7. PMID 1644362.
Hotez PJ, Pritchard DI (June 1995). "Hookworm infection". Sci. Am. 272 (6): 68–74. PMID
7761817.
Hotez P, Bethony J, Bottazzi ME, Brooker S, Buss P (2005) Hookworm: “The Great Infection
of Mankind”. PLoS Med 2(3): e67
Huttly, SRA. “The impact of inadequate sanitary conditions on health in developing
countries.” World Health Statistics Quarterly 43 (1990): 118-126.
James A. Phills et al., Pulmonary Infiltrates, Asthma and Eosinophilia due to Ascaris Suum
Infestation in Man, 286 New England Journal of Medicine 965-70 (1972)
Kasper, D.L., E. Braunwald, A.S. Fauci, S.L. Hauser, D.L. Longo, and J.L. Jameson, Eds.
2005. Harrison’s Principles of Internal Medicine: Enteroviruses and Reoviruses, page 114.
New York: McGraw Hill.
Kasper, D.L., E. Braunwald, A.S. Fauci, S.L. Hauser, D.L. Longo, and J.L. Jameson, Eds.
2005. Harrison’s Principles of Internal Medicine: Microbial Bioterrorism, p. 1284. New York:
McGraw Hill.
Keiser, Utzinger. (2008) Efficacy of Current Drugs Against Soil-Transmitted Helminth
Infections. JAMA 299(16):1937-1948
Jernigan, D.B., P.L. Raghunathan, B.P. Bell, et al. 2002. Investigation of BioterrorismRelated Anthrax, United States, 2001: Epidemiologic Findings. Emerging Infectious Diseases
8:1019–28.
Komar, N. 2003. West Nile Virus: Epidemiology and Ecology in North America. Advances in
Virus Research 61:185–234.
Larocque, Caspia, Gotuzzo, MacLean, Soto, Rahme, Gyorkos.(2006) A double-blind
randomized controlled trial of antenatal mebendazole to reduce low birthweight in a
hookworm-endemic area of Peru Tropical Medicine and International Health, 11(10):14851495
137
Loukas, Alex, Jeffrey M. Bethony, Susana Mendenz, et al. “Vaccination with recombinant
aspartic hemoglobinase reduces parasite load and blood loss after hookworm infection in
dogs.” PLoS Medicine 2 (2005): e295.
Loukas, Alex, Jeffrey M. Bethony, Angela L. Williamson, et al. “Vaccination of dogs with a
recombinant cysteine protease from the intestine of canine hookworms diminishes the
fecundity and growth of worms.” Journal of Infectious Diseases 189 (2004): 1952-1961.
Loukas, Alex, Jeffrey M. Bethony, Susana Mendenz, et al. “Vaccination with recombinant
aspartic hemoglobinase reduces parasite load and blood loss after hookworm infection in
dogs.” PLoS Medicine 2 (2005): e295.
Mabaso, Appleton, Hughes (2004) Necator Americanus transmission in inland areas of sandy
soils in KwaZulu-Natal, South Africa Tropical Medicine and International Health 9(4):471476
Massa K, Magnussen P, Sheshe A, Ntakamulenga R, ndawi B, Olsen A.(2009) ”The effect of
the community-directed treatment approach versues the school-based treatment approach on
the prevalence and intensity of schistosomiasis and soil-transmitted helminthiasis among
schoolchildren in Tanzania.” Trans R Soc Troop Med Hyg 103(1):31-7
Meadows, M. 2004. Project Bioshield: Protecting Americans from Terrorism. FDA Consumer
38:32–33
Moraes, L.R.S., J. Azevedo Cancio, and S. Cairncross. “Impact of draining and sewerage on
intestinal nematode infections in poor urban areas in Salvador, Brazil.” Transactions of the
Royal Society of Tropical Medicine and Hygiene 98 (2004): 197-204.
Morens, D.M., G.K. Folkers, and A.S.Fauci. 2004. The Challenge of Emerging and Reemerging Infectious Diseases. Nature 430:242–49.
Morse, S.S. 1995. Factors in the Emergence of Infectious Diseases. Emerging Infectious
Diseases 1:7–15.
Mwangi, Tabitha W., Jeffrey Bethony, and Simon Brooker. “Malaria and helminth
interactions in humans: an epidemiology viewpoint.” Annals of Tropical Medicine
Parasitology 100 (2006): 551-570.
Murdoch ME, Asuzu MC, Hagan M, et al. Onchocerciasis: the clinical and epidemiological
burden of skin disease in Africa. Ann Trop Med Parasitol. 2002;96:283–96.
National Institute of Allergy and Infectious Diseases. Factsheet: NIAID Research on Severe
Acute Respiratory Syndrome (SARS). http://www.niaid.nih.gov/factsheets/sars.htm. Accessed
on September 8, 2005.
National Institutes of Allergy and Infectious Diseases. Factsheet: NIAID Research on West
Nile Virus. http://www.niaid.nih.gov/factsheets/westnile.htm. Accessed on September 8,
2005.
Nordberg, E & Kingondu,T (2007). Communicable diseases (4th ed) Nairobi AMREF
138
Pal, Chattopadhyay, Sengupta (2007) A study on the prevalence of hookworm infection in
four districts of West Bengal and its linkage iwth anemia. Indian J Pathol Microbiol.
50(2):449-452
Pawlowski, ZS; Schultzberg K (1986). "Ascariasis and sewage in Europe". in Block JC.
Epidemiological Studies of Risks Associated With the Agricultural Use of Sewage Sludge:
Knowledge and Needs (EUR). Elsevier Science Pub Co. pp. 83–93. ISBN 1-85166-035-6.
Peters, C.J. 2005. Marburg and Ebola: Arming Ourselves against the Deadly Filoviruses. New
England Journal of Medicine 352:2571–73.
Peter J Hotez, Jeff Bethony, Maria Elena Bottazzi, Simon Brooker, and Paulo Buss,
"Hookworm: the Great Infection of Mankind", PLoS Med. 2005 March; 2(3): e67.
PLoS Medicine. 2005. The Global HIV/AIDS Vaccine Enterprise: Scientific Strategic Plan.
http://medicine.plosjournals.org/archive/1549-1676/2/2/pdf/10.1371_journal.pmed.0020025S.pdf. Accessed on September 8, 2005.
Ruebush, Mary. Why Dirt is Good: 5 ways to make germs your friends. Kaplan, 2009.
Salafsky B, Fusco AC, Li LH, Mueller J, Ellenberger B (October 1989). "Schistosoma
mansoni: experimental chemoprophylaxis in mice using oral anti-penetration agents". Exp.
Parasitol. 69 (3): 263–71. doi:10.1016/0014-4894(89)90072-6. PMID 2507345.
Stoll NR (1962) On endemic hookworm, where do we stand today? Exp Parasitol 12:241-252.
Spiegel, Andre, Adama Tall, Georges Raphenon, et al. “Increased frequency of malaria
attacks in subjects co-infected by intestinal worms and Plasmodium falciparum malaria.”
Strachan, David. “Hay fever, hygiene, and household size.” British Medical Journal 299
(1989): 1259-1260.
Strachan D P. (2006). "Hay fever, hygiene, and household size". BMJ. 18 (299): 1259–60.
PMID 2513902
Taylor L.H., S.M. Latham, and M.E. Woolhouse. 2001. Risk Factors for Human Disease
Emergence. Philosophical Transactions of the Royal Society B: Biological Sciences 356:983–
89.
The Gopu Berry p33. Part 4 School Journal number.2 1989 Dept of Education Wellington
N.Z.
John, David T., and William A. Petri. Markell and Voge's ical Parasitology. Philadelphia:
Saunders, 2006.
Transactions of the Royal Society of Tropical Medicine and Hygiene 97 (2003): 198-199John,
David T. and William A. Petri, Jr. Markell and Voge’s Medical Parasitology: Ninth Edition.
St. Louis: Saunders Elsevier, 2006.
139
UNAIDS. 2004. AIDS Epidemic Update. http://www.unaids.org/wad2004/EPI_1204_pdf_en/
EpiUpdate04_en.pdf. Accessed on September 8, 2005.
UNAIDS. 2004. UNAIDS at Country Level: Progress Report.
United States. Centers for Disease Control and Prevention. "Guidelines for the Investigation
of Contacts of Persons With Infectious Tuberculosis and Guidelines for Using the
Quantiferon –TB Gold Test for Detecting Mycobacterium tuberculosis Infection, United
States." MMWR 54(No. RR-17) 2005.
Ungchusak, K., P. Auewarakul, S.F. Dowell, et al. 2005. Probable Person-to-Person
Transmission of Avian Influenza A (H5N1). New England Journal of Medicine 352:333–40.
Vasundhra M., (2006). Community health nursing Jaypee brothers India
Verle, Kongs, De, Thieu, Depraetere, Kim, Dorny (2003) Prevalence of intestinal parasitic
infections in northern Vietnam Tropical Medicine and International Health 8(10):961-964
Williamson, Angela L., Paolo Lecchi, Benjmain E. Turk, et al. “A multi-enzyme cascade of
hemoglobin proteolysis in the intestine of blood-feeding hookworms.” The Journal of
Biological Chemistry 279 (2004): 35950-35957.
Williams-Blangero S, VandeBerg JL, Subedi J, et al. (April 2002). "Genes on chromosomes 1
and 13 have significant effects on Ascaris infection". Proc. Natl. Acad. Sci. U.S.A. 99 (8):
5533–8.
doi:10.1073/pnas.082115999.
PMID
11960011.
PMC:
122804.
http://www.pnas.org/cgi/pmidlookup?view=long&pmid=11960011.
Whipworm Infection. MedlinePlus Medical Encyclopedia. US Federal Government public.
domain. Update Date: 7/16/2004. Updated by: Daniel Levy, M.D., Ph.D., Infectious Diseases,
Greater Baltimore Medical Center, Baltimore, MD. Review provided by VeriMed Healthcare
Network.
Wood C.H., Glanville H. &Vaughan V.J., (1997). Community Health. AMREF. RegalPress
(R) ltd. Nairobi.
World Health Organization. Onchocerciasis and its control. Report of a WHO Expert
Committee on Onchocerciasis Control. World Health Organ Tech Rep Ser. 1995;852:1–104.
World Health Organisaion (2004). The world Health Report 2004 – Changing history.
http://www.who.int/whr/2004/en/. Accessed on September 8,2008.
WHO, Geneva, July 2008 Weekly Epidemiological Record, 2008, 83 27/28: pp 237–252.
WHO. “School deworming at a glance.” March 2003
WHO/GTP (1997). Global Tuberculosis control. Geneva: World Health Organisation.
Wu ML, Jones VA (January 2000). "Ascaris lumbricoides". Arch. Pathol. Lab. Med. 124 (1):
174–5.
PMID
10629158.
http://journals.allenpress.com/jrnlserv/?request=getabstract&issn=0003-9985&volume=124&page=174.
140
Yong, Tai-Soon, Jong-Ho Lee, Seobo Sim, et al. “Differential diagnosis of Trichostrongylus
and hookworm eggs via PCR using ITS-1 sequence.” Korean Journal of Parasitology 45
(2007): 69-74.
Zhan, Bin, Sen Liu, Samirah Perally, et al. “Biochemical characterization and vaccine
potential of a heme-binding glutathione transferase from the adult hookworm Ancylostoma
caninum.” Infection and Immunity 73 (2005): 6903-6911.
World Health Organization. 2004. The World Health Report 2004—Changing History.
http://www.who.int/whr/2004/en/. Accessed on September 8, 2005.
World Health Organization. 2005. Progress on Global Access to HIV Antiretroviral Therapy:
An Update on 3 by 5. http://www.who.int/3by5/fullreportJune2005.pdf. Accessed on
September 8, 2005.
World Health Organization. Smallpox Fact Sheet. http://www.who.int/mediacentre/factsheets/
smallpox/en/. Accessed on September 8, 2005.
World Health Organization. Poliomyelitis Fact Sheet. http://www.who.int/mediacentre/
factsheets/fs114/en/. Accessed on September 8, 2005.
World Health Organization. Roll Back Malaria. http://www.rbm.who.int/cmc_upload/0/000/
015/367/RBMInfosheet_6.htm. Accessed on September 8, 2005.
World Health Organization. Tuberculosis Fact Sheet. http://www.who.int/mediacentre/
factsheets/fs104/en/. Accessed on September 8, 2005.
World Health Organization. Influenza Fact Sheet. http://www.who.int/mediacentre/
factsheets/fs211/en/. Accessed on September 8, 2005.
World Health Organization. 2005. Avian Influenza: Assessing the Pandemic Threat.
http://www.who.int/csr/disease/influenza/WHO_CDS_2005_29/en/. Accessed on September
8, 2005.
World
Health
Organization.
Avian
Influenza.
avian_influenza/en/. Accessed on September 8, 2005.
http://www.who.int/csr/disease/
World Health Organization. SARS: Breaking the Chain of Transmission. http://www.who.int/
features/2003/07/en/. Accessed on September 8, 2005.
Yang H, S.K. Kim, M. Kim, et al. 2005. Antiviral Chemotherapy Facilitates Control of
Poxvirus Infections through Inhibition of Cellular Signal Transduction. The Journal of
Clinical Investigation 115:379–87.
141