* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Methylcyclohexane + bromine and heat = 1-bromo-1
George S. Hammond wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hydroformylation wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Homoaromaticity wikipedia , lookup
Aromaticity wikipedia , lookup
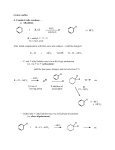
CH 335, ORGANIC CHEMISTRY II FINAL EXAM * March 14, 2004 ANSWER KEY grading notes in red answers in blue. 250 points even 1. Given below are the NMR, GC-MS, and FTIR data for Compound V. After examining it carefully, provide a structure and give a NAME for Compound V. Please justify your deduction of the structure. (16 points; 10 for structure, 6 for data analysis) 1H NMR (in CDCl3): 7.1 (1H, triplet); 6.7 (1H, dd); 6.5 (1H, dd); 6.3 (1H, dd); 3.6 (2H, s); 2.3 (3H, s). (values in ppm; dd=doublet of doublets.) 13C NMR (in CDCl3): (proton-decoupled) 148, 139, 129, 120, 118, 111, 21. (values in ppm.) FTIR (neat liquid): major bands at 3433, 3353 (not broad), 2947, 2920, 1537, 1278, 1312, 1293, 775. Many smaller bands below 1500. (values in cm-1) GC-MS: highest peak at 108 (intensity 2%), most intense peak at 107, relatively intense peak at 106 (85%). Many very small fragments below 106, but none over 3% intensity. (values in m/z.) Proton NMR – benzene ring. Double doublets indicate asymmetric placement of two or more different substituents. Carbon NMR – 21 indicates alkyl group (perhaps 2.3 singlet is a methyl?) FTIR – 3433/3353 cm-1…an alcohol? Amine? GC-MS – odd mass number probably means nitrogen. Must be an amine. METHYL GROUP ; AMINO GROUP…….3-aminotoluene (or any number of other names including m-toluidine) fits the data best. Or – you can do a calculation from 107 m/z and find molecular formulas that might fit the data, but this would be tedious. If o-aminotoluene was given, only 1 point was deducted if the data analysis was good. In fact, the o isomer will have very similar spectra (the proton NMR does have differences enough to tell them apart, of course, but that is beyond the scope of this problem.) As much as 4 points was deducted if appropriate comments were not made on the data. 2. Provide names (common or IUPAC), or a complete structure (showing all bonds) as appropriate, for the four compounds shown below. Parts E and F are optional. Parts A, B, E, and F must be exactly correct in order to receive points. (24 points) A. 2-isobutyl-4-p-toluenesulfonylanisole (4 points); OMe O-SO2 Full credit given if you only had two oxygens on the sulfur. B. (9S, 7E) –9-cyano-10-cyclohexyl-6,6-diiodo-7-decen-4-ynol (4 points); See my homework for chapter 13. There was one almost exactly like this. If you gave the ( R ) isomer, you got -1. If you gave the ( Z ) isomer, you got -1 FOR PARTS C and D, alphabet was not considered. C. (6 points) O2N O CH3 NO2 ; trans (or 1R, 2S) – 2’,4’-dinitrophenoxy – 2 – methylcyclohexane OR as an ether – trans-2’-methylcyclohexyl-2,4-dinitrophenylether NOTE: Stating the 1R, 2S stereochemistry is really the right way to name this compound. However, in light of the intensity of this exam and the fact that R and S stereochemistry were not emphasized greatly this term, trans was accepted this one time. ________________________________________________________________________ (name as an aliphatic compound) D. (12 points) CH2Br H3C O ; 6 points each; although if you had one completely correct name and one with errors, you got +8 for the correct one and the incorrect one was only graded out of 4 points. 4-bromomethyl-2-methyl-1-oxacyclopropylbenzene ________________________________________________________________________ (name as an aromatic) 4’-bromomethyl-2’-methylphenyloxacyclopropane/ethylene oxide ________________________________________________________________________ (name as a substituted epoxide/oxacycloalkane, or alkene) E. N,N-diperdeuterophenyl-deuteroformamide (4 points Ex Cr); D O D D D D N D D D D D D +4 or +0 F. the haloalkane DDT (4 points Ex Cr). See lecture notes; I drew this many times over a period of 2 weeks. +4 or +0 3. Provide explanations for FOUR of the following observations (but no more than ten words per response; use structures wherever possible, instead of words). Or, use structures to validate the statements. All five can be done for extra credit, but indicate which one is being done for bonus points. [16 points; 4 each] NOTE: I use more than 10 words in my responses for the benefit of this answer key! These can all be described in under 10 words, however. For full credit, you had to include the key points listed below. A score of 0 to 4 points was awarded based on correctness. A. The chloromethylbenzenes (mono, dichloro, and trichloro) will usually, but not always, direct electrophiles to the meta position. Not much data exists for monochloro/dichloromethylbenzenes and their direction of electrophiles to o,p or m positions. However, just on the basis of dipole direction, it would seem plausible that they would direct mainly to the m position. Trihalomethylbenzenes always direct m because the trihalomethyl group has no electron-donating propensity that would ordinarily direct an electrophile to o,p. B. Nitrosobenzene (shown below) is a deactivating o,p-director. N=O Like the halogens, NO is polarized in the direction out of the ring, so it is a deactivating substituent. However, the lone pair on N will stabilize the intermediates and direct exclusively o,p. C. A student used trimethylphosphine instead of triphenylphosphine for a Wittig reaction in attempt to make ethylenecyclopentane (be careful! ethylene is not the same as vinyl!) . The reaction failed. The methyl group protons will react with sodium amide, right along with the ethyl group that was intended for acid/base reaction to prepare the correct alkene. The reaction still goes to completion, but it will give a mixture of products. D. A chemist was preparing to carry out a hydroboration of an alkyne. He first rinsed out the flask with methanol and water. The next morning he came in, assembled the reaction apparatus and flask, and added B2H6 to the flask. Then something horrible happened, before the alkyne could even be added. What happened? Give at least one balanced reaction to support your answer. The flask had water and methanol in it. The slightest trace of ROH/HOH will incite a violent reaction with borane, which is a Lewis acid. If borane is inactivated, it can’t react with the alkyne. Borane loses a hydride and methanol loses the H of the OH group, to give hydrogen gas (a violent explosion, if in sufficient molar quantity) BH3 + CH3OH = CH3OBH2 + ½ H2 + lots of heat 4. Evaluate the validity of each of the following statements. If the statement is valid, give an example to elucidate the fact. If the statement is invalid, correct it to make it valid or provide a reason as to why it is invalid. All five can be done for extra credit, but be sure to indicate which one is being done for bonus points. [16 points, 4 each] A. 1-chloro-2-nitrobenzene reacts much slower than does 4-chloroaniline in a nucleophilic aromatic substitution mechanism. INCORRECT. The nitrobenzene reacts much faster because of the EWG nitro groups, allowing the nucleophile easy admission onto the ring. The aniline does not react in N.A.S. mechanisms at all due to the electron-donating amino group. B. Phenyl groups can undergo very close interaction in proteins partially because a momentary dipole can be induced in the benzene ring. VERY CORRECT – although hard to picture unless you are a biochemist (as stated in lecture notes) The stacking interaction between two or more phenyl groups is substantial and will influence the structure of the protein. C. The reversible addition of water to alkenes can take place by a Bronsted basecatalyzed process as well as an acid-catalyzed process, depending on the substrate. CORRECT. Any time there is an electron-stabilizing group (carbonyl, imino, etc.), a polar species with a free electron pair can add to the double bond. D. When 4-chlorobutylbenzene is exposed to a Lewis base such as AlCl3, the AlCl3 ensures that no rearrangement occurs and that that intermolecular Friedel-Crafts reaction proceeds normally. INCORRECT. The statement is completely nonsensical. AlCl3 is a catalyst for the reaction. Rearrangement does occur (the butyl group undergoes rearrangement) and the AlCl3 doesn’t prevent it…actually, it promotes it in the same way it promotes a non-rearranged F-C reaction. 5. Provide synthetic sequences that show how to make each of the following. Please do not give any mechanisms. (34 points) A. trans-6-methyl-2-heptene. Re-read the name and draw the structure out first. As starting materials, you may use acetylene and any compound that has 4 carbons or less, plus any organic/inorganic reagents. (8 points) Acetylene + NaNH2 = acetylide ion. Add to isobutyl bromide…get substituted alkyne. Treat with strong base again, add methyl iodide. Remove inorganic byproducts (NaI, NaBr, etc.) Reduce with Li/NH3 to get trans C=C bond. +6 if the reduction wasn’t done with Li/Na/K and NH3. B. For the ether shown below. As starting materials, you can use benzene and methylcyclohexane, plus any organic/inorganic reagents. (12 points) O2N O NO2 CH3 **It was announced during the exam that you can start with methylcyclohexene. If you started all the way from methylcyclohexane, you got an additional 2 points. Hopefully, by now, everyone knows that a simple two-step process can convert an alkane to an alkene (E1 or E2). Methylcyclohexane + bromine and heat = 1-bromo-1-methylcyclohexane Add KOtBu – get methylcyclohexene. Treat with borane/THF – get antiMarkovnikov trans alcohol (mix of diastereomers.) Deprotonate alcohol and react with 1-chloro-2,4-dinitrobenzene to get the product (NUCLEOPHILIC AROMATIC SUBSTITUTION!!!). To make the aforementioned benzene, you need to start with benzene, add chlorine, then nitrate with nitric/sulfuric acids. React with the alcohol and get the product shown. Then separate the trans isomers. There are other ways to make this product, but given the starting materials, this is pretty much the only way. The borane/THF step is important if you want the stereo-correct product; -2 points for using a different method. No points deducted for not separating the trans isomers (R,R) and (S,S). C. For the following trisubstituted benzene, starting from ethylbenzene and any compound with 1 carbon or less, plus any organic/inorganic reagents. (14 points) CH2Br H3C O Two methyl groups, one of which is brominated – so must put the non-brominated one on last. Ethylbenzene + NBS = bromoethylbenzene. KOtBu to complete the elimination (giving vinylbenzene). Then…one of two ways. 1. Epoxidize first with MCPBA and then CH3Cl/AlCl3 (the 3-membered ring is probably large enough that you will get mostly para), then NBS to brominate the methyl, then CH3Cl/AlCl3 again. [If you went this route, you got 12 points] OR 2. CH3Cl/AlCl3, then NBS, then CH3Cl/AlCl3 again, and finally, epoxidize with MCPBA. This second way is probably better. The reason is, ethylene oxide is very reactive and can undergo reactions with Lewis acids like AlCl3 and some solvents. It’s better to make epoxidation the last step. [If you went this route, you got 14 points.] 7. Write mechanisms for each of the following, using the usual meticulously correct electron pushing we have been discussing for the last two terms. Neatness counts, and be sure to include ALL nonbonding electron pairs and formal charges. Not doing either or both will result in penalty! Also include all resonance forms for any intermediates. (48 points) Up to ½ of the points for each part were deducted for bad electron pushing, lack of formal charges in intermediates, etc. The severity of the errors determined how many points were taken off (omission of steps usually resulted in more points taken off.) A. (i) the acid-catalyzed addition of methanol to isobutene (CH2=C(CH3)2) (10 points); Break C=C bond; add H+ to the methylene carbon, to give t-butyl carbocation. SN1 addition of MeOH solvent molecule to the vacant p orbital on the central C; loss of H+ to any available conjugate base. The addition of MeOH occurs in Markovnikov fashion, adding to the more-substituted carbon. (ii) the anti addition of X-Y to isobutene (assume that X is more electronegative than Y) (10 points); Break X-Y bond; Y is less e.n. so adds to the C=C of isobutene, identical to the mechanism we know for adding halogens. X (now negatively charged) will add from the opposite side of the bond, to the C bearing the two methyls (in Markovnikov fashion “add to the most”). B. the para-nitration of isopropylbenzene (10 points); See the lecture notes; no one should have lost any points on this question. C. syn and anti elimination of HBr by KOtBu/HOtBu from the following compounds (please also indicate which mechanism, syn or anti, is likely to predominate for both molecules): (i) trans-1-bromo-2-methylcyclohexane (8 points; show one mechanism for SYN and another for ANTI) FOR SYN: The bromo and methyl groups are on opposite sides, both axial. The H on the carbon with methyl is SYN to the bromo. So, the larger lobes of the sp3 orbitals are on the same side of the C-C bond. Bromide leaves, H+ leaves, with the electrons making the new pi bond. (This doesn’t happen > 90% of the time.) FOR ANTI (preferred): The bromo is axial; the methyl is still axial, BUT, instead, we’re considering the H+ on the OTHER carbon next to the bromo, which is also axial. Usual mechanism involves loss of H+ and bromide. The sp3’s are ANTI to each other, with one large lobe pointing “down” and the other large lobe pointing “up”. For both, the H+ is lost to t-butoxide. (ii) cis and trans-1-bromo-1-pentene (8 points; just show SYN for cis isomer and ANTI for trans). Br H base H H Br H The top molecule is trans; the bottom is cis. T-butoxide removes the beta-H+ on each. This reaction is, for some reason, not very stereospecific, as the textbook indicates. The reaction rate for the trans alkene is only slightly higher than that for the cis; both give the same product in good yield. D. (10 points) Cyclopentadienone + acetylene benzene + carbon monoxide In case you’re interested, this was one of the early methods used to prepare benzene on an industrial scale. NOTE: Be sure to clearly indicate which electrons are being pushed to which carbons. You may want to mark the carbons with X, , etc. Diels-Alder reaction for first step; break the 2nd pi bond of acetylene and break both pi bonds in cyclopentadienone, form new sigma bonds and make new pi bond on the c-pentadiene ring (4n + 2 cycloaddition) - EASY PART. HARD PART: need to lose carbon monoxide. SO – break one of the bridgehead bonds – electrons flow towards the O and :C≡O: is released; and have the other bridgehead bond break, with the electrons going back into the 6-membered ring. The existing ring pi bonds will shift initially but once the aromatic structure is made…that’s it, you’re done. 8. Complete the following reactions, showing or indicating stereochemistry for those indicated with a ; only give the major product where requested. If not specified otherwise, give structures of ALL products formed. (72 points; 6 points each). For part C, if you can’t do the third step fully, just indicate generally what the outcome of the total sequence of reactions will be. A. On-Bu Hg(OAc)2 n-Butanol +2 for giving vicinal ether; OXYMERCURATION/DEMERCURATION ETHER SYNTHESIS B. excess HBr, peroxide, heat Br Br +4 for giving monobromide only. ANTI-MARKOVNIKOV HBr ADDITION C. OH sodium amide 1-chloro-3-methyl-2-butene heat O CH3 OH the allyl group can go here too ACID/BASE (1st step) ; Claisen Rearrangement (2nd step.) There was a homework problem like this one – we have not studied the mechanism of Claisen Rearrangement yet, but the solutions manual gives a pictorial illustration of how this unique synthetic method gives a high yield of the vinylic product. If you got to the ether, you got +2. If you gave the allyl phenols (or one of them), you got full credit. D. Cl Al2Cl6 MAJOR PRODUCT +2 for giving any of the minor products. FRIEDEL-CRAFTS ALKYLATION E. D2, palladium, charcoal the D’s usually end up cis to each other. cis-1,2-dideuterio-bicyclo-[2.2.2]-2-methyloctane is the product. DEUTERIUM ALKENE REDUCTION F. OH OtBu 1) K2Cr2O7, acid 2) tBuOCH2CHPPh3 +6 or +0 WITTIG RXN. G. SCH3 NC cis-2-pentene 1. CN-Br 2. NaSCH3 (and also the enantiomer) +4 for giving the trans product. +2 if bromide is in the product and stereochem is correct. ANTI X-Y ADDITION/SN2 H. O O Cl2, FeCl3 +6 or +0 Cl CHLORINATION (electrophilic) MAJOR PRODUCT I. I D-I (gas phase) D (stereoisomeric mix) +6 or +0 (no need to comment on stereochem); 1,4-ADDITION OF DX J. O hot, acidic KMnO4 OH OH + CO2 OH O O +5 if CO2 is not listed; STRONG OXIDATION K. O3 Zn, HOAc O O H O + H H O +5 if stereochem on C6 is wrong; +3 if the methyl is not on C6; +0 if the connectivity of the starting material is incorrect (i.e., methyl not on C1); OZONOLYSIS L. O O MgBr H+, HOH Hg 2+ (acidic) HO **Hg2+ (acidic) = HgSO4/H2SO4/HOH GRIGNARD/ALKYNE OXIDATION (MARKOVNIKOV); if the mercury reaction is incorrect but the first step is correct, +2 points. 9. Shown below are twelve structures. Circle all those which are AROMATIC. (12 points) If CORRECT reasoning was given, it was applied to extra credit. Circling wrong product/not circling correct product was a simple –1 point. YES – aromatic by resonance. By pushing the pi electrons joining the two rings, you can get a double-aromatic species (cyclopentadienyl anion/cyclopropyl cation). NO. The sp3 nonbonding electrons on O ruin it. N O CH2 YES. YES; the B doesn’t change anything B YES. 6 pi electrons The N is still sp2 YES 6 continuous pi electrons NO – the carbon with NO2 is sp3 O H + N+ O O2N NO; broken by 2 sp3 carbons. However, the individual components can be viewed as three individual systems (by definition, 4n + 2 gives an aromatic structure.) NO – although all the C-O bonds have pi character; plus, this is a resonance form (and they don’t really exist!) O O O NO; broken by sp3 methyl YES – the –CH2- group lies above the pi system, making the 10 pi electron system aromatic. There was a question in the problem set with the all-cis isomer of this compound. YES. The 6 pi electron system remains intact 10. The 2-propenyl (allyl) system is an important one in organic chemistry. Provide the molecular orbitals of the allyl anion, cation, and radical (with appropriate +/- signs), and indicate the electronic configurations of each. Indicate the carbons that are electron-rich in each. (12 points) 9 points total for the electronic configurations (with nonbonding, antibonding, bonding orbitals correctly drawn). 3 points total for indicating the electron rich carbons. SEE THE ORBITAL DIAGRAMS AND OCCUPANCIES FOR ANION, CATION, AND RADICAL IN CHAPTER 14. You were expected to pretty much reproduce these here. EXTRA CREDIT (46 points) – partial credit not freely awarded: 11. Go back to question #8 and classify each reaction by type (i.e., Markovnikov addition, etc., but don’t use the term ‘redox’). Write answers in the space to the LEFT of each lettered part. (12 points.) SEE ALL-CAPS ABOVE 12. Go back to the non-aromatic structures shown in question #9 above and indicate why they are not aromatic. (6 points, but don’t assume six incorrect structures). SEE COMMENTS ABOVE ALIPHATICS 13. This term, we learned about two classes of compounds, ____________________ and AROMATICS ______________________. We had three distinct “learning modules” this term. In ten words or less, describe all three modules. Last term, two of our modules were “Alkanes and Radical Halogenation” and “Chemistry of Alcohols”. (8 points) 1. SPECTROSCOPY/STRUCTURE DETERMINATION 2. ALKENES AND ALKYNES, AND THEIR THERMODYNAMICS 3. AROMATIC RING CHEMISTRY (If you listed thermo as a separate category, that was fine too) To get 8 points extra credit, you had to have everything listed here. 14. Compound M has molecular formula C8H6. It has two 13C NMR absorptions, and two 1H NMR singlets (one at 4.0 ppm, the other at 1.0 ppm, but not equally intense). In the GC-MS, a large abundance of a structure with m/z = 26 was observed. Propose a structure for M. (10 points) (SOMETIMES THE CORRECT ANSWER IS CLOSER TO YOU THAN YOU’D THINK! – BACK IN #11). I ALSO ADDED ON THE OVERHEAD PROJECTOR THAT A LARGE ABUNDANCE OF A SIX-CARBON SPECIES WAS OBSERVED. +10 or +0 extra credit. 15. Bromocyclohexane was run in NMR at 77K and at 298K. Describe the differences in each spectrum. Hint: Use chair conformations to explain. (10 points) The axial H’s and equatorial H’s will have different shifts at 77 K. At 298 K, the rapid equilibrium between the two forms (with axial and equatorial bromo) causes the spin states to average to zero, and thus, no splitting is observed. At 77 K, splitting between H’s is clearly observed. For 10 points, you pretty much had to state the above. If you showed differences in the chair structures, but didn’t comment on splitting, you may have gotten 2 points extra credit. Have a peaceful and restful spring break!