* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download H CH 4 Homework
Canonical quantization wikipedia , lookup
Hidden variable theory wikipedia , lookup
EPR paradox wikipedia , lookup
Renormalization wikipedia , lookup
History of quantum field theory wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Ferromagnetism wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Double-slit experiment wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Tight binding wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Particle in a box wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Matter wave wikipedia , lookup
Atomic theory wikipedia , lookup
Atomic orbital wikipedia , lookup
Hydrogen atom wikipedia , lookup
Wave–particle duality wikipedia , lookup
Electron configuration wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
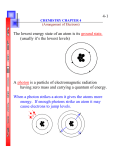
H CH 4 Homework Problems: 1. Calculate the frequency of electromagnetic radiation with a wavelength of 1.2 x 10-12 m. 2.5 x 1020 Hz 2. Orange light has a wavelength of about 600 nm. What frequency would it have? 5 x 1014 Hz 3. A typical radio wave has a frequency of 100 MHz. How much energy does it have? 6.626 x 10-26 j 4. Write an equation that relates energy and wavelength. E = hc/ 5. What is the difference in energy between the orange light in number 2 and blue light that has a wavelength of about 420 nm? 1.42 x 10-19 j 6. Provide electron configurations for the following. a) Na – 1s2 2s2 2p6 3s1 b) Zn – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 c) Br – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 d) U – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 7s2 5f4 e) Cs – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1 7. Provide orbital notation for the following. a) K – _ 1s 2s 2p 3s 3p 4s b) S - 1s 2s 2p 3s 3p c) Ti - 1s 2s 2p 3s 3p 8. Provide dot notation for the following. a) Li b) F c) O d) Mg 4s _ __ __ __ 3d Review: 1. Write and label the equation that relates frequency and wavelength. c = 2. Define: a) electromagnetic radiation any wave that travels at the speed of light b) wavelength distance from 1 point on one wave to the same point on the next wave c) frequency number of “things” that happen in a given period of time d) quantum specific, finite amount of energy e) photon light “particle” 3. What is meant by the dual wave-particle nature of light? Light can act as a wave and a particle 4. Describe the Bohr model of the atom. Nucleus with specific quantized energy orbits where an electron can be found. 5. Write and label the equation that relates energy and frequency. E = h 6. Distinguish between the ground state and the excited state. Ground state is the initial state of the electron. Excited state is after it absorbs energy 7. According to Bohr, how is a line spectrum produced. Electron falls from the excited state and emits a photon 8. What is the major difference between the quantum and the Bohr model of the atom? In the Bohr model the electron can be pinpointed. 9. What is the principle quantum number? How is it symbolized? Main energy level around the nucleus. Symbolized with numbers 1,2,3,… 10. What information is given by the orbital quantum number? List the 4 orbital quantum numbers in order of increasing energy. Shape of the region of probable location…shape of the standing wave. S,p,d,f/0,1,2,3 11. What information is given by the magnetic quantum number? Explain the notation for distinguishing among the different p orbitals. Orientation of the orbital about the nucleus. X,y, and z orientations. / +1,0,-1 12. What information is given by the spin quantum number? Direction the generated magnetic field takes in an applied magnetic field. 13. What is stated in the Aufbau principle? Order in which the orbitals are filled from lowest to highest energy. 14. What is stated in Hund’s rule? Each orientation of an orbital receives 1 electron before any receives a second. 15. State the Pauli Exclusion Principle. What is the significance of the spin number? No 2 electron in the same atom can have the same set of quantum numbers. Differentiates between 2 electrons in the same orientation of the same orbital. 16. How would 2 electrons in the same orientation of the same orbital compare in terms of the values for their four quantum number? Same principle, orbital, and magnetic but different spin. 17. What is meant by the highest occupied energy level? The highest principle quantum number with electrons. 18. Determine the highest occupied energy level for the following: a) He 1 b) Al 3 c) Ca 4 19. Given the electron configuration for element Z as 1s2 2s2 2p5 : a) How many electrons are in an atom of Z? 9 b) What is the atomic number of Z? 9 c) What is the highest occupied energy level? 2 _ 1s 2s 2p e) Write its electron dot notation. F d) Write its orbital notation. 20. Identify the following element: 1s2 2s2 2p6 3s1 Na 21. When sodium is heated, a yellow spectral line with an energy of 3.37 x 10-19 J is produced. What is the frequency of the light? 5.09 x 1014 1/s What is its wavelength? 5.90 x 10-7 m 22. Determine the frequency of light with a wavelength of 4.257 x 10-7 cm. 7.05 x 1016 1/s 23. How much energy is given off by an electron when it falls from the 5th energy level to the first in the hydrogen like atom. -2.09 x 10-18 j 23. Assign quantum number using the n,l,m,s system to electrons in the 4th energy level of arsenic.