* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Sophisticated sperm analysis ever required?
Survey
Document related concepts
Transcript
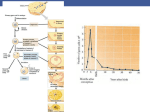
"Minimal workup before infertility treatment" ---"Sophisticated sperm analysis ever required?" Donald P. Evenson, Ph.D., HCLD, Department of Chemistry and Biochemistry, South Dakota State University and SCSA Diagnostics, Inc., Brookings, SD 57006. E-mail: [email protected] INTRODUCTION Because couples are tending to wait before trying to start a family, they are motivated to seek infertility treatment more quickly, being prompted by the general recognition that women will have suffered a significant reduction in their fertility potential by the time they turn 40. However, there is new evidence (Singh et al, 2003; Evenson et al, unpublished) to suggest that men can also suffer an age-related reduction in fertility, although this is much more heterogeneous. Since the landmark papers of Macomber and Sanders in 1929 and MacLeod in 1951, male fertility diagnosis has been strongly based on the concept that sperm concentration, motility and morphology are primary determinants for male factor reproductive success. A typical initial infertility screening will include a WHO-type assessment of the man’s semen for these parameters (Rowe et al, 1994). Even so, it has been recognized by many that the WHO “normal” or “reference” values have a significant weakness, namely the significant overlap between “fertile” and “infertile”. Thus, a normal semen analysis (SA) result cannot guarantee that a man’s sperm are functionally capable of moving through the female reproductive tract or of fertilizing an egg. Conversely, a man with an abnormal SA may be fertile. While the SA is a first step in the work-up of male fertility potential, it should be accompanied by a physical examination, hormonal evaluation and sperm function testing – since sperm fertilizing capacity depends on more than just the number of sperm per milliliter of semen. A great deal of emphasis has been placed on sperm morphology assessment in the past, and to an even greater degree today using the so-called “strict criteria” (Kruger et al, 1986). It has been questioned whether a method that considers every “defect” (small, doubtful or large) equally important for classifying a sperm cell as “not normal” has sufficient power of analysis (Niederberger, 2003). Yet, 1 a number of papers show that very low sperm morphology (<5%) is associated with failed fertilization for routine IVF (Guzick et al, 2001). Thus, a strict morphology analysis could be considered to be a minimal work-up since it allows physicians to consider more effective therapeutic interventions for male infertility. It is recognized that ~50% of couples presenting with “couple infertility” will have a male factor infertility component, while in 30 to 40 % of couples the infertility will be caused only by male factors. With this recognition, the couple and physician need to determine the best course of action that is compatible with the couple’s financial and personal perspectives. It is the view of (perhaps) a diminishing number of experts that the most productive and cost-effective therapy can be delivered only after a complete evaluation of the man (Comhaire et al, 1987; Cummins and Jequier, 1994; Rowe et al, 1994; Mortimer, 2000; Brugh et al, 2003). The amount of pre-treatment investigation that the couple will agree to undertake depends on the couple’s perceived urgency to achieve a pregnancy, as well as upon a combination of other factors such as finances, insurance coverage, religious or cultural background and personal beliefs. Similarly, the level of pre-treatment investigation offered depends on factors such as the philosophy of the clinic and the training and expertise of the physicians and staff. Listed below are the levels of work-up for male infertility evaluation that may be offered: 1. Couple “checking fertility factors” for future. The man may have a routine SA to evaluate sperm count, motility and morphology, to assess the likelihood of achieving natural conception. 2. Couple trying for a natural pregnancy for >1 year. By definition, this couple is now labeled as “infertile” but they might not be. Assessment will include a routine SA and other more comprehensive tests including Sperm Penetration Assay (SPA), acrosome function, antisperm antibodies, viability of sperm with time and DNA fragmentation test. Treatments can then be designed to bypass functional problems that were identified by the work-up (Mortimer, 2000). In order of intervention, these are: pericervical artificial insemination of sperm (the simplest 2 intervention); intrauterine insemination (IUI) to bypass problems of cervical sperm transport; routine IVF to bypass more severe problems of sperm transport or poor sperm quality; and intracytoplasmic sperm injection (ICSI) to bypass all considerations of sperm physiology and function by delivering the male partner’s haploid genome contribution directly into the egg. Epididymal or testicular sperm recovered surgically from men with azoospermia can also be used in ICSI. 3. Couples trying for one year with one or more miscarriages. Genetic disorders may lead to varying degrees of male subfertility or infertility (Brugh et al, 2003). These may be characterized as: karyotypic abnormalities, which are more common in infertile men than in the normal population (5.8% cf. 0.5%; Johnson, 1998); deletions within chromosomes that regulate spermatogenesis; specific mutations within genes; or DNA fragmentation. Klinefelter syndrome (XXY–XXXXY karyotypes) is the most common sex chromosome disorder and in one study occurred 30 times more frequently in infertile men attending an infertility clinic (De Braekeleer and Dao, 1991). The incidence of karyotypic abnormalities and Y-chromosome microdeletions increases as the sperm count decreases and it has been recommended that these be evaluated in all men with sperm counts <5 million/ml, before attempting assisted conception procedures (Foresta, 2001). DNA fragmentation, thought to be random breaks in nuclear DNA (not discounting hot spots), has been shown in numerous reports to be highly correlated with infertility. 4. Couples with “idiopathic infertility”. This label implies that the couple has been thoroughly investigated for the cause of their infertility. Depending upon the age and motivation of the couple, they will likely proceed with the ordered sequence shown in option 2 (above) and finally ICSI. ICSI In the ICSI procedure, one entire sperm is inserted into an egg. It is thought that only two features of the entire sperm are needed for ICSI success: the paternal haploid genome with intact DNA and a normal haploid complement of 23 chromosomes; and the proximal centriole that serves as the paternal 3 centrosome. Given that the centriole to centrosome conversion is normal, then the all-important factor for embryo development and normal adult life is a normal paternal genetic package. ICSI is considered as a last resort by some clinics, while in others the management program goes straight to ICSI, avoiding the costs of male testing. This is often the case if the man has poor semen quality, such as low sperm count and/or poor motility, viability and morphology, which reduces the chance of conceiving naturally. ICSI is also used when the man has antisperm antibodies or a sexually transmissible disease, such as HIV. When ICSI was introduced more than a decade ago, it was thought that perhaps all male infertility issues could be solved as long as the man had any sperm. The thought persisted that once the sperm had fertilized the egg then embryo development would proceed normally. However, this is obviously not the case since ICSI pregnancy rates are well below 100%. Even so, there has been a trend within some countries to forgo all testing on the male (other than to determine if sperm exist), and to treat all infertility cases using ICSI, without extensive testing of the male. Such views have been labeled as “insidiously lazy” (Cummins, 1999) and “a dangerous loss of control over the clinical decision-making process” (Jequier and Cummins, 1997). An alternative view, as stated by Mortimer (2000), is for structured management strategies that use diagnostic information to recognize causative factors amenable to simpler, even systemic, therapies with reasonable chances of pregnancy. This approach does not resort prematurely to assisted reproduction technology, and represents a more rational, costeffective approach to infertility management. In this approach, ICSI represents a last resort to be used when less-invasive, lower-cost treatments have been deemed inappropriate or have failed. NEW CONCEPTS FOR PATIENT INFERTILITY MANAGEMENT A relatively new clinical concept for patient infertility management is sperm DNA fragmentation. It has long been known that exposure to agents such as high levels of ionizing radiation (Sailer et al, 1995; Amadi and Ng, 1999) or DNA alkylating agents (Evenson et al, 1993) cause poor reproductive outcome including infertility, spontaneous miscarriages and birth defects, due to DNA damage in genes 4 essential for normal offspring. However, the idea of fragmented DNA in sperm of normal adults and men with sub/infertility had not been seriously considered. Indeed, in a review of a dozen recentlypublished books for the lay person on “how to get pregnant”, there was not a single paragraph dedicated to this topic, and even in professional books and clinical articles the topic was rarely considered until about 5 years ago. In 1980, we published the first report showing a direct relationship between sperm DNA fragmentation and fertility status in men and bulls (Evenson et al, 1980), using a new technique later called the Sperm Chromatin Structure Assay (SCSA; Ballachey et al, 1988). This original study showed that the susceptibility of sperm DNA to partial denaturation was highly correlated with fertile/infertile status. It is now known that this DNA denaturation occurs at sites of DNA strand breaks (Aravindan et al, 1997; Tritle and Evenson, unpublished), data supported by two other techniques developed a decade later, the Comet and TUNEL assays. A number of papers have correlated the extent of DNA strand breaks measured by the Comet (Morris et al, 2002) and TUNEL assays (Aravindan et al, 1997) with attempts to learn the origin of DNA damage (Sakkas et al, 1999) For the SCSA (Evenson et al, 1999; 2002), sperm are treated with a low pH buffer (pH 1.2) for 30 s and then stained with acridine orange (AO). The low pH partially denatures the DNA at sites of a single- or double-stranded DNA break. The AO intercalates into the DNA, and upon exposure to a 488 nm laser beam fluoresces green when associated with dsDNA, and red when associated with ssDNA. Fig 1 shows two SCSA cytograms, one with a high DNA fragmentation index (DFI) and another with a high DNA stainability (HDS). Each of the 5000 dots represents a single sperm and its position on the cytogram is determined by the amount of green and red fluorescence. FIG 1 AND LEGEND. Several reports have described light microscope observations of a significantly higher ratio of red/green sperm in infertile patients. However, the problem of fluorescent fading and loss of equilibrium AO staining on a glass slide precludes its use for reliable counseling of individual couples, 5 although it can provide an overall picture of the status of DNA fragmentation in a sperm population (see cover of Science, Evenson et al, 1980). Following the first description of a correlation between DNA fragmentation and fertility potential in animal and human sperm, two more papers were published on the DNA integrity status of cancer patients pre- and post-cancer treatment. The data were very heterogeneous, so we decided to perform some animal dose-response reproductive toxicology studies to evaluate whether the SCSA test was statistically quantitative. Impressively, the data showed a very high dose response level of increased red fluorescence. Also, repeat measures on the same sperm sample had a CV of only ~1 % or less and the standard deviation among animals (rats, mice, rabbits) was very low, thus showing high intra- and inter-individual variability, with good reproducibility (example: Evenson et al, 1989). The latter is due to the use of a flow cytometer that easily measures at least 5000 sperm per sample using objective machine criteria, rather than the human eye with the light microscope. Extensive data were also published on animal fertility and SCSA results (Ballachey et al, 1988), which showed correlations up to 0.98 (P<0.001) for bulls. The very significant advantage of the SCSA over the Comet assay and the light microscope TUNEL assay is the power of the flow cytometer to measure 5–10x106 sperm rather than the 100–200 examined light microscopically. Although TUNEL measured by flow cytometry has the same power as the SCSA, only the SCSA can detect the percentage of sperm that have immature chromatin. The effect of using SCSA as a “sophisticated measure” of sperm DNA for structured management of infertile couples in different clinical situations is presented below: (a) SCSA never ordered The advantages of the decision to ignore SCSA are that neither the patient nor the physician needs to learn about the test and its implications, and the patient saves the ca. $200 fee. However, there are several disadvantages to this decision: 6 Despite normal SA results, the patient could still have a high rate of sperm DNA fragmentation (DFI) leading to sub/infertility of unknown etiology (Larson et al, 2000; Saleh et al, 2002; LarsonCook et al, 2003; Virro et al, 2004). There may be no impetus to relate the sub/infertility to prior exposures to reproductive toxicants. The patient may not be made aware that lifestyle factors may be contributing to his sub/infertility. It has been suspected for years that many substances, occupations and prescribed medications may affect semen quality, and relationships have been shown between poor semen quality and exposure to pesticides, organic solvents, heat and heavy metals. However, it now appears that lifestyle choices including smoking, heavy alcohol consumption and excessive use of hot tubs, as well as high fever can significantly increase DNA fragmentation (Potts et al, 1999; Evenson et al, 2000). This can result in the production of sperm that can fertilize an egg but contribute a defective genome for embryo development. (b) Use SCSA in cases of failed IVF The advantages of this approach are. Even though IVF failure is multifactorial, one of the reasons for failure, even when “high quality” day 3 embryos are transferred, is sperm DNA fragmentation. If SCSA-defined damage is found in >30% of sperm, present data suggest that the pregnancy failure is likely due to a damaged sperm genome. Failed IVF fertilization may be due to a high level of SCSA-defined high DNA stainable (HDS) sperm, i.e. sperm nuclei with immature chromatin. Semen samples with >15% HDS sperm have been associated with reduced pregnancy rates in an in vivo study (Evenson et al, 1999) and with reduced fertilization by IVF (Virro et al, 2004). The best course of action if a positive result is obtained is to determine lifestyle factors that may have contributed to DNA fragmentation, and change them if possible. (c) Use SCSA for all men with risk factors for DNA fragmentation 7 Risk factors include abnormal SA, varicocele, cancer and cancer treatment. For men with abnormal SA, SCSA results could potentially be improved by lifestyle changes, in particular taking antioxidants. Varicocele repair has been shown to cause dramatic improvement in SCSA results (e.g. 45% DFI to 22% DFI), although there may have also been lifestyle factors that contributed to the improved DFI. At least six publications concerning SCSA and/or TUNEL measures show effects of both cancer and cancer treatment on elevated DFI (Hiroshi et al, 2001). The first paper published by our group showed that some, but not all, men with testicular cancer had high DFI levels prior to further cancer treatment (Evenson et al, 1984). Other studies have found that young men presenting with Hodgkin’s disease often show levels of DFI that border on or are above the 30% threshold for statistical probability of reduced fertility (Fossa et al, 1997). Thus, when oncologists suggest storage of cryopreserved sperm prior to chemotherapy, they must recognize that some patients already have such a high DFI they may decide it is not worth the expense or the wait to start treatment. There is no real disadvantage to this approach, except for those who would prefer to repeat IVF/ICSI without looking for any causes or possible improvements. (d) Order SCSA in all cases with unexplained infertility, regardless of SA results There is a solid advantage in this approach, since there is clear evidence that despite excellent SA results there can still be high levels of sperm DNA fragmentation (Twigg et al, 1998). An article in press (Virro et al, 2004) reports on 12 couples in a study of about 250 couples, with prolonged idiopathic infertility. It was found in each of these couples that the man had SCSA-defined DFI values >30%, and all 12 decided to use donor sperm. Within three months 9 couples had conceived. The evidence very strongly suggests that the cause of the idiopathic infertility was as “simple” as the man having viable, good looking and motile sperm that carried a genetic package that was so damaged it could not support a viable pregnancy. 8 Often, by the time a couple has arrived at the classification of unexplained infertility, they may well have spent a $100,000 and a great deal of emotional and physical pain in their quest for a successful pregnancy. If the likely etiology is sperm with fragmented DNA, would it not be worth a couple of hundred dollars to learn this early and move on to donor sperm? In the light of this consideration, there is no disadvantage to ordering the SCSA in all cases of unexplained infertility. (e) The SCSA should be ordered on all infertile couples and used for all sperm donors. The advantage of this rationale in the clinic that first attempts to determine the etiology of the male factor infertility, rather than going straight to IVF, is that the SCSA can provide an important piece of the puzzle as to the cause(s) of infertility. Some clinics use the SCSA on all new IVF/ICSI referrals, and if the DFI is >30%, one additional embryo is transferred with the rationale that at least 1/3 of the sperm are genetically compromised to the extent of a high likelihood of at least one embryo dying. Some clinics have used the SCSA as part of their screening and evaluation of couples’ chances of conceiving and have used this information in their shared risked program. Other clinics struggling with a low success rate being reported to SART at the CDC, have used the SCSA in attempt to reduce the number of patients more likely to fail in their program. Screening by sperm banks is relatively intense, since they only want to accept sperm from those men with a very high probability of producing successful full-term pregnancies. Some scientists (e.g.., Rupert Amann, symposium talk) have advocated that all potential sperm donors be tested for fragmented sperm DNA, since high levels may reduce the chance of a pregnancy and could also increase the likelihood of transmission of damaged DNA leading to pathologies such as early childhood leukemia. The disadvantage to this approach is the term “all”, which for a medical procedure/diagnosis is very likely an overstatement. It is possible that there are many arguments, albeit not obvious at this time, as 9 to why this should not be considered. However, with published studies using SCSA, COMET and TUNEL, AO and nick translation assays and clinical trials all concluding that sperm DNA fragmentation is correlated with taking a longer time to in vivo pregnancy, with more IVF/ICSI cycles, with an increased spontaneous miscarriage rate or with no pregnancy at all, the time has come for physicians and patients to add this test into their search for the etiology of male factor infertility. CONCLUSION The “minimal” but unsophisticated workup for the male partner of a suspectedly infertile couple is the more-than-half-century-old routine light microscope parameters of sperm count, motility and morphology. Yet these parameters will not predict male factor fertility success. Beyond the routine SA, a great amount of effort has gone into sperm function tests as well as body physicals and analyses of hormone levels. The term “sophisticated” implies measures that are new in design and use new, often high tech, instruments that can measure some factor not obtained by easy methods. New methods under development include things like “sperm DNA on a chip” (Chan et al, 2002) for detecting genetic anomalies, but these are not ready for routine clinical application. Even karyotyping and FISH analysis are too labor-intensive to be part of the routine SA. The SCSA is an example of a “sophisticated” test being used in infertility clinics today. All papers published on the relationship between sperm DNA fragmentation and reproductive outcome support the utility of the SCSA for detecting defects not seen by light microscopy. As other tests come along, the above concepts of “no use”, or “use at certain stratified levels of infertility outcomes”, can be used to determine their value to the fertility clinic and its patients. REFERENCES Ahmadi A, Ng SC. (1999) Fertilizing ability of DNA-damage spermatozoa. J. Exp. Zool. 284:696-704 Aravindan GR, Bjordahl J, Jost LK and Evenson DP (1997) Susceptibility of human sperm to in situ DNA denaturation is strongly correlated with DNA strand breaks identified by single-cell electrophoresis. Exp. Cell Res. 236:231-237. 10 Ballachey BE, Saacke RG and Evenson DP (1988) The sperm chromatin structure assay: Relationship with alternate tests of sperm quality and heterospermic performance of bulls. J. Androl. 9:l09-115. Brugh VM III, Matschke HM and Lipshultz LI (2003) Male Factor infertility. Endocrinol Metab. Clin N. Am. 32: 689-707. Chan PJ, Mann SL, Corselli JU et al (2002) A simple DNA disc chip in a microarray design based on modified comparative genomic hybridization for sperm DNA analysis. Fertil. Steril. 77: 1056-1059. Comhaire FH, de Kretser D, Farley TMM (1987) Towards more objectivity in diagnosis and management of male infertility. Results of a World Health Organization Multicentre Study. Int. J. Androl. 10:1-53. Cummins JM (1999) Potential pitfalls in male reproductive technology. In Gagnon, C. (ed.), The Male Gamete: From Basic Science to Clinical Applications. Cache River Press, Vienna, IL, 417-427. Cummings J, Jequier A (1994) Treating male infertility needs more clinical andrology, not less. Hum. Reprod. 9: 1214-1220. De Braekeleer M, Dao TN (1991) Cytogenetic studies in male infertility: a review. Hum. Reprod. 6:245-250. Evenson DP, Baer RK and Jost LK (1989) Long term effects of triethylenemelamine exposure on mouse testis cells and sperm chromatin structure assayed by flow cytometry. Environ. Mol. Mutagen. 14:79-89. Evenson DP, Darzynkiewicz Z and Melamed MR (1980) Relation of mammalian sperm chromatin heterogeneity to fertility. Science 240:1131-1133. Evenson DP, Jost LK, Corzett M and Balhorn R (2000) Characteristics of human sperm chromatin structure following an episode of influenza and high fever: a case study. J. Androl. 21: 739-746. Evenson DP, Jost LK and Gandy JG (1993) Glutathione depletion potentiates ethyl methanesulfonateinduced susceptibility of rat sperm DNA denaturation in situ. Reprod. Toxicol. 7:297-304. Evenson DP, Jost LK, Zinaman MJ, Clegg E, Purvis K, de Angelis P and Clausen OP (1999) Utility of the sperm chromatin structure assay (SCSA) as a diagnostic and prognostic tool in the human fertility clinic. Hum. Reprod. 14:1039-1049. Evenson DP, Klein FA, Whitmore WF and Melamed MR (1984) Flow cytometric evaluation of sperm from patients with testicular carcinoma. J. Urol. 132:1220-1225. Evenson DP, Larson K and Jost LK (2002) The sperm chromatin structure assay (SCSATM): clinical use for detecting sperm DNA fragmentation related to male infertility and comparisons with other techniques (Andrology Lab Corner) J. Androl. 23: 25-43. 11 Foresta C, Moro E and Ferlin A (2001) Y chromosome microdeletions and alterations of spermatogenesis. Endocr.Rev. 22:226-239. Fossa SD, De Angelis P, Kraggerud SM, Evenson D, Theodorsen L and Claussen OP (1997) Prediction of post-treatment spermatogenesis in patients with testicular cancer by flow cytometric sperm chromatin structure assay. Comm in Clinical Cytometry 30:192-196. Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajkima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA, Xu d, Vogel DL (2001) Sperm morphology, motility and concentration in fertile and infertile men. N. Engl. J. Med. 345:1388-1393. Hiroshi K, Larson K, Sharma R, Nelson D, Evenson D, Hiroshi T, Thomas A Jr and Agarwal A (2001) DNA damage in cancer patients before treatment as measured by the sperm chromatin structure assay. Fertil. Steril. 75: 469-475. Jequier AM and Cummins JM (1997) Attitudes to clinical andrology: A time for change. Hum. Reprod. 12:875-876. Johnson MD (1998) Genetic risks of intracytoplasmic sperm injection in the treatment of male infertility: recommendations for genetic counseling and screening. Fertil. Steril. 70:397-411. Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, van Zyl JA and Smith K (1986) Sperm morphologic features as a prognosis factor of in vitro fertilization. Fertil. Steril. 46:1118-23. Larson-Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET and Evenson DP (2003) Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil. Steril. 80: 895-902. Larson K, De Jonge C, Barnes A, Jost L and Evenson D (2000) Sperm Chromatin Structure Assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum. Reprod. 15: 1717-1722. Macomber D, Sander MB (1929) The spermatozoa count: its value in the diagnosis, prognosis and treatment of sterility. N Engl. J. Med. 200:981 MacLeod J (1951) Semen quality in 1,000 men of known fertility and 800 cases of infertile marriages. Fertil. Steril. 2:115 Mortimer D (2000) The future of male infertility management and assisted reproduction technology. Hum. Reprod. 15: 98-110. Morris ID, Ilott S, Dixon L and Brison DR (2002) The spectrum of DNA damage in human sperm assessed by single cell electrophoresis (COMET assay) and its relationship to fertilization and embryo development. Hum. Reprod. 17:990-998. Niederberger C (2003) Responses to semen analysis CART report. J. Androl. 24:329-331. 12 Potts RJ, Newbury CJ, Smith G, Notarianni LJ and Jefferies TM (1999) Sperm chromatin changes associated with male smoking. Mutat. Res. 423:103-111. Rowe PJ, Comhaire FH, Hargreave TB et al (1994) WHO manual for the Standardized Investigation and Diagnosis of the Infertile Couple. Cambridge University Press, Cambridge, UK. Sailer BL, Jost LK, Erickson KR, Tajiran MA and Evenson DP (1995) Effects of X-ray irradiation on mouse testicular cells and sperm chromatin structure. Environ. Mol. Mutagen. 25:23-30. Sakkas D, Mariethoz E, Manicardi GC, Bizarro D, Bianchi PG, and Bianchi U (1999) Origin of DNA damage in ejaculated human spermatozoa. Rev. Reprod. 4:31-37. Saleh RA, Agarwal A, Nelson DR, Nada EA, El-Tonsy MH, Alvarez JG, Thomas AJ Jr., Sharma RH (2002) Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil. Steril.78:313-318. Singh F, Muller CH and Berger RE (2003) Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil. Steril. 80:1420-1430. Twigg JP, Irvine DS and Aitken RJ (1998) Oxidative damage to DNA in human sperm does not preclude pronucleus formation at intracytoplasmic sperm injection. Hum Reprod.13:1864-1871. Virro MR, Larson-Cook KL and Evenson DP (2004) Sperm chromatin structure assay (SCSA®) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil. Steril. 81(5): in press. 13 14 15