* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download TOTAL PARENTERAL NUTRITION
Survey
Document related concepts
Transcript
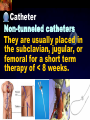
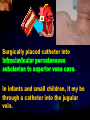
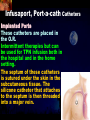
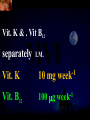
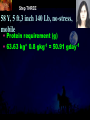
Total Parenteral Nutrition Basic Components Prot./Carb./Fat /Elect./ Trace/ Vit. Stability and Compatibility Drug-Nutrient Interactions Temperature and pH Labeling Sterility & Stability Storage and Packaging Infusion Pumps & Filtering Laboratory Monitoring Compliance Adjusting Therapy The pharmacist initiating, maintaining, and monitoring the therapy’s affect on the patient’s metabolic condition. Assess the stability and the compatibility of the parenteral nutrition solution. Pharmacist-Physician- Nurse-Dietitian • In 1960s Drs. Wilmore and Dudrick researched on central venous for growth in infant, elderly patients with catabolic medical conditions Originally termed hyperalimentation • Replaced with TPN, which is more descriptive of the technique Psychological Impacts • Its horrible watching others eat when you cannot. • Feelings of dependency and immobility can occur. • Can be countered by intermittent or nocturnal feeding. Indicated when adequate nutrition cannot be maintained via GIT. Nasogastric Tube Indications of TPN Carcinoma extensive burns Geriatric refuse to eat Young anorexic patients Surgical patients who should not be fed orally [NPO] GIT motility disorder Severe vomiting, when enteral feeding cannot be tolerated TPN IV administration of calories, nitrogen and all other nutrients in sufficient quantities to achieve tissue synthesis and anabolism. Peripheral Parenteral Nutrition Nutrients are supplied via a peripheral vein, usually a vein in the arm. Another term for PPN is peripheral venous nutrition (PVN) Peripheral venous nutrition (PVN) PVN is used when a patient is unable to ingest adequate calories enterally or when central venous nutrition is not feasible. Concentration 4.25% amino acid+ 10% dextrose IV fat emulsion should be run simultaneously with the PVN to minimize thrombophlebitis. Urine 1.5 L Perspiration 1.0 L. =Average compensation of water should be 2.5 L daily. Four methods to calculate fluid requirements include: 1234- 1500mL for first 20 kg body weight, then 20mLkg-1 1500mLm-2 30mLkg-1 of body weight 1.2 mLKcal-1 TPN Solutions To provide essential nutrients in normal daily fluid requirements [3L]. Hypertonic solutions -1 (>2000 mOsmL ) is needed. To minimizes vascular damage and risk of phlebitis or thrombosis, TPN solutions must be infused into a large diameter central vein, with rapid dilution by high blood flow. TPN Infusion The amino acids + dextrose solution with additives is mixed in a one bag per day system. The fat emulsions are a separate solution. Starting and weaning the TPN should be done gradually. The starting rate should be no more than 50 mLhr-1 for 4-6 hrs. TPN Infusion The rate can be increased 25% every 4-6 hrs. Weaning is accomplished by decreasing the rate by 25% every 4-6 hrs. Blood glucose monitoring is recommended every six hrs after TPN initiation. Catheter These catheters are usually divided into: 1- Non-tunneled catheters, 2- Tunneled catheters, and 3- Implanted ports. Catheter Non-tunneled catheters They are usually placed in the subclavian, jugular, or femoral for a short term therapy of < 8 weeks. Insertion of these catheters should be done with sterile technique. Chest X-ray to assures the correct placement of the catheter and the absence of insertion complications. The tip of the catheter must be in the superior or inferior vena cava. Catheter Non-tunneled catheters They are usually placed in the subclavian, jugular, or femoral for a short term therapy of < 8 weeks. Catheter Non-tunneled catheters Insertion of these catheters should be done with sterile technique. Chest X-ray to assures the correct placement of the catheter and the absence of insertion complications. The tip of the catheter must be in the superior or inferior vena cava. Surgically placed catheter into infraclavicular percutaneous subclavian to superior vena cava. In infants and small children, it my be through a catheter into the jugular vein. Hickman®, Groshong, Broviac®, Catheters Tunneled Catheters Long term therapy > 8 weeks. They are placed in the in the O.R. These catheters are made of . The catheter is placed in the chest area, tunneled under the skin and enters a large vein and then is threaded into the superior vena cava. Infusaport, Port-a-cath Catheters Implanted Ports These catheters are placed in the O.R. Intermittent therapies but can be used for TPN infusion both in the hospital and in the home setting. The septum of these catheters is sutured under the skin in the subcutaneous tissue. The silicone catheter that attaches to the septum is then threaded into a major vein. Dressing Changes and Catheter Care Central venous dressing should be changed at least once a week or more frequently based on patient condition/ need. Initial dressing change should be completed within 48 hours after placement of catheter. Medical aseptic technique is absolutely essential in the management of TPN. Components of TPN •Fluids •Carbohydrate as dextrose (3.4 kcal/g) •Protein as amino acids (4 kcal/g) •lipids (10-11 kcal/g) •Electrolytes •Vitamins •Trace minerals Components of TPN Dextrose and lipids to provide energy. 70%-85% of calories from dextrose Protein for tissue synthesis and repair. 15%-30% from lipids. utritional assessment Determine the appropriate amount of calories needed for the patient by assessing height, weight, ideal body weight and % of weight loss. • Calculation of patient requirements calculated using Harris-Benedict • basal requirement- 25kcal/kg body weight DESIGNING THE TPN FORMULA Estimate Basal Energy Expenditure (BEE) Harris-Benedict equation: • For women the formula is: = 655.1 + (9.56 * weight in kg) + (1.86 * height in cm) - (4.68 x age) • For Men: = 66.67+ (13.75 x weight in kg) + (5 x height in cm) - (6.76x age) Total Daily Expenditure(TDE) • TDE= BEE* Activity*Stress • Activity • BED=1.2 • Ambulatory=1.3 • • • • • Stress: Surgery: 1.2 Infection: 1.4-1.6 Trauma: 1.3-1.5 Burns: 1.5-2.1 • non-stressed (ambulatory)30 kcal/kg body weight • mild stress (malnourished)35-40kcal/kg body weight • severe injury or sepsis- 4560kcal/kg body weight • severe burns- up to 80kcal/kg body weight • infants up to 200kcal/kg body weight Standard TPN Solution Final concentration :4.25% aa 25% dex. Calorie: nitrogen ratio is 125:1. Additives Electrolytes are included when ordering the standard TPN solution. It is possible to order an electrolyte-free solution and then order the more appropriate electrolytes for the patient. Total Parenteral Components Macronutrients Non-Protein Calories Carbohydrate - dextrose Fat IV Long Chain Fatty Acids Micronutrients Electyrolytes - Na, K, Ca, Mg, P, Cl, acetate Vitamins - all accept K and B12 which are given separately intramuscular Trace Metals - Zn, Cu, Cr, Mn (No Fe) Carbohydrate Requirements • 3.4 kcal/g • Enternal nutrition: 4.0 kcal/g 0.8 g/kg/day = unstressed patient 1.0 g/kg/day = mildly stressed patient 1.2 g/kg/day = renal dialysis 1.5 g/kg/day = Moderately stressed 2.0 g/kg/day = Severely steressed 3.0 g/kg/day = Burned patient INSOLUBLE UNDIGESTED IN BLOOD. 15-24 g of nitrogen -1 day g. SYNTHETIC AMINO ACIDS 6.25 g. usable Nitrogen Protein- requirements usually estimated empirically: non-stressed 0.5-1g/kg mild stress 1.2-1.4 g/kg moderate stress 1.5-2.0g/kg severe stress 2.0-2.5g/kg Protein Solutions Standard formulas EAA (40%) and NEAA (60%) available as 3-15% solutions Protein Solutions Renal formula provides only EAA Protein Solutions Hepatic Formula Protein Solutions Stress Formula provides highest valine, isoleucine, leucine Amino Acid Solutions Protein is provided as a crystalline amino acid solution. 500 ml bottles are standard. Solutions vary in amino acid concentration and amino acid composition. | SYNTHETIC AMINO ACIDS FREAMINE TRAVASOL AMINOSYN arbohydrates Carbohydrates Hydrous Dextrose (glucose) Provides 3.4 kcalg-1 1 L D5W =170 kcal, 1 L D25W = 850 kcal Final dextrose concentrations 5-10% (peripheral) 35% (central) D5W=252 mOsmL-1, D25%=1263 mOsmL-1 INTRAVENOUS LIPIDS Fat Emulsions Only O/W emulsions can be given by IV. After 2 weeks of TPN Dry scaly skin, hair loss, impaired wound healing Fat provides 9 kcal/g Components soybean (50% linoleic) safflower (72%) glycerol water egg yolk phospholipid Fat Emulsion 10% Actions Indications Dosage Drug Incompatibilites Fatty acids in To prevent ADULTS: 100 Do not add any emulsion form fatty acid mg/min for the other medication used as a deficiency for first 15-30 min to the infusion. source of patients then increase calories and to requiring to 2-3 ml/min if provide parenteral no reaction. essential fatty nutrition, and Give only 500 acids to reverse a ml (50 gm) first known 24 hrs, in no deficency state reaction characterized increase by scaly skin following day. Do not exceed 2.5gm/kg/day INTRAVENOUS LIPIDS • Intravenous lipids have the highest caloric density of any components of parental nutrition • Intralipid is composed of soybean oil, egg yolk phospholipids, and glycerol. The major fatty acids are linoleic 54%, oleic 26%, palmitic 9% and linolenic 8% Linoleic acid If a patient has been on TPN for 2 weeks Dry Scaly Skin, Hair loss, Impaired Wound healing. Soybean-oil emulsion (Intralipid) Safflower-oil emulsion (Liposyn) Essential Fatty Acids 500 mL BIW or TIW Emulsion Destabilization of TPN Creaming–accumulation of particles at the top of the emulsion. Aggregation–clumping of triglyceride particles within the emulsion. Coalescence–fusion of small triglyceride particles into larger particles. Cracking–separation of the oil and water components of the emulsion. Electrolyte Sodium Potassium Guidelines for Electrolyte Requirements Electrolyte Sodium Potassium Chloride Magnesium Calcium Phosphorus Amount/1000 Calories 40-50 mEq 30-40 mEq 40-50 mEq 8-12 mEq 2-5 mEq 15-25 mEq Recommended Daily Adult Doses of Parenteral Trace Elements Trace Element Dose Zinc 2.5-4.0 mg Copper 0.5-1.5 mg Chromium 10-15 ug Manganese 150-800 ug Selenium 40-80 ug A D E Niacin B1[Thiamine] B2[Riboflavin] C Panthenol acid MVI 2 5 mg 8000 U 800 U 4 U 80 mg 40 mg 8 mg 400 mg mg Folic Vit. K & . Vit B12 MVI separately I.M. Vit. K 10 mg -1 week Vit. B12 100 g -1 week TPN Interventions Warm to room temp 1 hr prior to use Hang TPN alone Dextrose concentration > 10% given through a central line Change TPN bag and filter every 24 hours Ordering and Mixing PN Solutions The physician writes the Rx TPN prescription. The pharmacist mixes the TPN solution using aseptic technique. Prescriptions are compounded by mixing the solutions at a 1:1 dextrose-to-amino acid ratio and placing in 1-L bags. Alternatively, lipids can be mixed with the dextrose/amino acid solution, referred to as the 3-in-1 total nutrient admixture (TNA). Administration TPN should always be given via an infusion pump. The pharmacist may be consulted regarding drug compatibility for simultaneous administration of two or more drugs through a single lumen of the catheter. Avoid the administration of blood products into the lumen designated for parenteral nutrition. Heparin Flush When parenteral nutrition infusion is being cycled, a heparin flush is needed to maintain patency of central venous catheter when solution is not infusing. Initial Considerations TPN infusion should start slowly so that the body has time to adapt to both the glucose load and the hyperosmolarity of the solution, and to avoid fluid overload. A pump controls the infusion rate of the TPN solution. There are specific steps in the inititiation procedure to follow regarding the initiation of TPN infusion. • Infusion Pumps: • Electronic ambulatory infusion pumps are commonly used in the home setting. • These pumps are lightweight and portable and can be programmed to deliver continuous infusions, intermittent infusions or single dose medications. • Many pumps have the capacity to taper the rate of an infusion. • Multichannel pumps allow for the administration of several different infusions at one time. General PN Initiation Procedures Start with 1 L of TPN solution during the first 24 hours (42 mL/hr as a start rate) Increase volume by 1 liter each day until the desired volume is reached Monitor blood glucose and electrolytes closely Pump administer TPN at a steady rate Don't attempt to catch up if administration gets behind. Continuous vs. Cyclic cyclic TPN the patient is fed at night. Cyclic TPN helps prevent hepatotoxicity that can develop with long-term TPN and the fasting period allows essential fatty acids to be released Renal failure Nephramine Balance of both essential and nonessential amino acids. Nephramine contains only essential amino acids Recommended only to decrease net urea synthesis for short periods of time. Close monitoring of serum ammonia levels is important. Hepatamine liver failure Patients with chronic liver disease are usually malnourished. Complications: GIT bleeding and infection. Standard TPN should be used if the patient does not have hepatic encephalopathy. can be used with patients with hepatic encephalopathy. It is a liver-specific amino acid mixture. Novamine Catabolic patient Metabolic respone to injury, burn or sepsis generates a neuroendocrine response that induces hypermetabolism, proteolysis, insulin resistance with hyperglycemia, and a depletion of lean body mass. In a catabolic state nutritional support is extremely important. Patients should be fed within 48-72 hours of insult to optimize the patient’s metabolic state. Nutritional requirements should be calculated. The optimal protein requirement of a critically ill patient is 1.5 to 2.0 gm/kg/day. NITROGEN BALANCE = Protein intake in gram - UUN + 3 6.25 UUN = urine urea nitrogen Novamine is a high concentration amino acid which can be used for the catabolic patient with a fluid restriction. Glucose metabolism can be altered in the critically ill patient. Metabolism of glucose can increase CO2 production and O2 consumption. The use of IV fat emulsion as the main calorie source is encouraged in these patients. Mechanics of Administering Titrate up slowly to allow pancreas to adapt to hypertonic dextrose load Give 1/3 of max rate on day 1, 2/3 on day 2 and full infusion on day 3 Taper to allow pancreas to adapt to withdrawal of hypertonic dextrose Infuse D10 if TPN abruptly discontinued Use filters (0.22 ). Fat can’t run through filters CLEAN ROOM • The clean room is a limited-access area, which is separated from the other pharmacy operations to minimize the potential for contamination. All products are prepared using the Class 100 laminar flow cabinets IV Admixture Environment • To provide sterility and pyrogen-free, proper environment is a must • Prepare admixture under laminar-flow filter • Air filter through High Efficiency Particulate Air (HEPA) flowing at 90fpm and remove 99.97% particles of 3 . • Air flow either horizontal or vertical • HEPA filter must be replace every 6 months • Technician or operators must wash hands, gloved and require gowning • Options For Compounding : • Manual or gravity preparation involves the use of transfer sets and syringes to separately add components to the empty container. • Automated compounders are more commonly used to admix components under computer-assisted control. Lipids can then be added to some of these products. The Automix pumps dextrose, amino acids, water, or fat, into an empty polyvinyl chloride bag The Micromix adds electrolytes and trace elements to the parenteral nutrition solution Withdraw additional additives manually Eight Steps Harris-Bendict equation TDE G of Amino-acids # of Kcal supplied by aminoacids 30% of daily Kcal Lipids G of lipids G of Carb. Daily fluid requirement Calc pp 220 Step One 58 Y, 5 ft,3 inch 140 Lb, no-stress, mobile • For women the formula is: = 655.1 + (9.56 * weight in kg) + (1.86 * height in cm) - (4.68 x age) = 655.1 + (9.56 * 63.63 kg) + (1.86 * 160 cm) - (4.68 x 58)= 1289.62 Kcal Step Two 58 Y, 5 ft,3 inch 140 Lb, no-stress, mobile • For women the formula is: = 655.1 + (9.56 * weight in kg) + (1.86 * height in cm) - (4.68 x age) = 655.1 + (9.56 * 63.63 kg) + (1.86 * 160 cm) - (4.68 x 58)= 1289.62 Kcal TDE= 1289.62 Kcal * 1.3= 1676.5 Kcal Step THREE 58 Y, 5 ft,3 inch 140 Lb, no-stress, mobile • Protein requirement (g) • 63.63 kg* 0.8 gkg-1 = 50.91 gday-1 Step Four 58 Y, 5 ft,3 inch 140 Lb, no-stress, mobile • Protein requirement (Kcal) • = 50.91 gday-1* 4 Kcalg-1= 203.64 Kcalday-1 Step Five 58 Y, 5 ft,3 inch 140 Lb, no-stress, mobile Lipid requirement (Kcal) 35% = 1676.5 Kcal* 0.35 = 502.95 Kcal Step six 58 Y, 5 ft,3 inch 140 Lb, no-stress, mobile Lipid requirement (g) =502.95 Kcal* 1g lipid /11 kcal = 45.72 g lipid Step Seven (1) 58 Y, 5 ft,3 inch 140 Lb, no-stress, mobile Fluid Requirement (mL) 30 mL* 63.63 kg/kg = 1909.09 mL Step Seven (2) 58 Y, 5 ft,3 inch 140 Lb, no-stress, mobile Fluid Requirement (mL) 1.2 mL* 1676.5 Kcal= 2011.8 mL Step Seven(3) 58 Y, 5 ft,3 inch 140 Lb, no-stress, mobile Fluid Requirement (mL) 1500 mL* 50 kg+( 13.63kg* 30 ml) = 1908.09 mL Step Seven (4) 58 Y, 5 ft,3 inch 140 Lb, no-stress, mobile BSA= (63.63)0.425*(63*2.54)0.725*0.007184 =1.66 m2 Fluid Requirement (mL) 1500 mL* 1.66 m2= 2490 mL Step Seven 58 Y, 5 ft,3 inch 140 Lb, no-stress, mobile Fluid Requirement (mL) 2490 mL+1909.09 mL+ 1908.09 mL + 2011.8 mL/4= • Temperature and pH: • Temperatures below freezing or above room temperature may result in destabilization of the lipid emulsion. • pH below 5.3 or the addition of additives with a pH of 5.0 may also destabilize the emulsion. • Temperature & calcium-phosphorus stability. • As the temperature increases, there is an increase in the rate of dissociation of calcium and phosphorus from their salts. This allows more free calcium and phosphorus to be precipitated. • Labeling: • The American Society of Enteral and Parenteral Nutrition (ASPEN) addressed the issue of standard labeling for PN solutions in its recent guidelines. Labels for PN admixtures should include amount per day of base formula, electrolyte additives, micronutrients and medications, quantity per liter for those who admix in 1L volumes, and dosing weight. Auxiliary labels may be helpful when PN orders are written in a different format than the standard label. • Storage and Packaging: • TPN solutions should always be transported and stored under controlled-temperature refrigeration. • TPN solutions are delivered from the pharmacy to the patient’s home, a cooler with cooler blocks should be used. • Refrigerators should be checked to make sure the temperature is constant and that adequate space is available for storing PN solutions and supplies. • Filtering: • Use of a filter during the administration of PN solutions may prevent complications arising from any particulate matter, microprecipitates or microorganisms potentially present. • A 0.2 -1.2-m filter should be used for TPN solutions with amino acids and dextrose. • Filters should be replaced every 24 hours. • A clogged filter, indicates some type of problem with the TPN solution, such as contamination of the solution, precipitation, cracking or incompatibilities. What happens if a patient misses a dose? TPN treatment is set at a specific time on a regular basis. If the patient misses his or her treatment, take a soon as possible. * You should never double up on doses at the same time* Monitoring Blood work must be drawn to establish baseline lab values, which include: electrolytes, creatinine, triglycerides, BUN, phosphorous, glucose, albumin, magnesium, CBC + differential, carbon dioxide, and total protein Monitoring Blood work must be drawn to establish baseline lab values, which include: electrolytes, creatinine, triglycerides, BUN, phosphorous, glucose, albumin, magnesium, CBC + differential, carbon dioxide, and total protein Thereafter, monitoring can be performed 2-3 times per week. •other include body weight and temperature. Monitoring of the TPN Patient Recommended patient care monitoring Vital signs every eight hours Intake and output Daily weights Blood sugars every six hours until the patient’s glucose is stable and then at least every day. Baseline Monitoring Considerations The initial TPN prescription is based only on estimates of the patient's kcalorie, protein, and micronutrient needs. The patient's weight, protein, and micronutrient status must be monitored to ensure the prescription's adequacy. Ionized Ca++ = (measured serum Ca) + [4.0 - actual albumin (g/dL)] x 0.8 AAssessment considerations include: •Maximum weight gain in anabolism is 1/4 to 1/2 pound per day. More than that indicates fluid retention. •Adjust calcium lab values in hypoalbuminemic patients as follows: Monitoring of the TPN Patient Acute condition, unstable patient, early nutrition support Electrolytes, BUN, SCr: 3-7 times per week Calcium, magnesium, phosphate: 1-3 times per week LFT’s, TP, ALB: once weekly or every other week Triglycerides: weekely or as appropriate for IV fat emulsion use. Monitoring of the TPN Patient Stable hospitalized patient, prolonged parenteral nutrition support Electrolytes, BUN, SCr: 1-3 times per week Calcium, magnesium, phosphate: once weekly or every other week LFT’s TP, ALB: every 2-4 weeks CBC/ differential, PLC RBC indices: every 2-4 weeks • Daily • • • • Body weight Vital Signs Fluid intake Nutritional intake • Output (urine, other losses) • Serum Electrolytes (Na, K, Cl, HCO3, BUN, Creatinine) • Weekly • • • • Albumin Total Protein Transthyretin Liver Association Test (AST, ALT, Alkaline Phosphate, GGT, Bilirubin, Nitrogen balance) Monitoring Measurement Normal value Comments Total iron binding capacity 250 - 450 mcg/dL Reliable measures of visceral protein status Iron 20 -160 mcg/dL Transferrin saturation 20%-45% Albumin 3.7 - 5.2 g/dL Markers of nutritional status Saturation levels less than 15% indicate iron deficiency Monitoring Electrolytes Normal value Comments Phosphate 2.5 - 5.0 mg/dL Excess or rapid initiation of TPN may result in severe hyperphosphatemia. Magnesium 1.6 - 2.4 mEq/L Serum concentrations may not reflect intracellular deficiencies or losses Calcium 4.4 - 5.1 mEq/L Long-term TPN may result in osteoporosis if losses are not replaced Sodium 135 -147 mEq/L Excessive fluid loss without adequate replacement or fluid deficits may cause abnormalities in serum sodium Potassium 3.5 - 5.0 mEq/L Severe malnourishment results in depletion of intracellular potassium stores; renal failure or excess administration may cause hyperkalemia Monitoring Glucose 65 - 115 mg/dL Hyper- or hypoglycemia are common complications of PN. Blood glucose should be checked daily, 1hr after infusion is stopped CBC and differential Deficiencies of trace elements or vitamins may result in anemias Carbon dioxide 21 - 31 mEq/L Excess bicarbonate losses may result in acidosis; vomiting, loss of gastric fluids or prolonged use of diuretics may cause alkalosis BUN 8 - 18 mg/L Serum creatinine 0.6 - 1.2 mg/L Urinary output Used to monitor a patient’s renal and fluid status. Daily temperature Used to monitor for infection Weekly weights Useful as a marker for nutritional goals Complications Infection Infection can occur at the PN catheter insertion site. Dressings must be changed daily. Technical Complications Technical complications include pneumothorax and hemothorax that can result if the chest wall is perforated with catheter needle. Metabolic Complications A number of metabolic complications can occur. Infectious Complications • Two sources of infectious complications are: • catheter-related and • solution infusate contamination • Catheter-related remain the most serious infection • Infusate is not very common, but admixture of intravenous fluid must follow strict guidelines to prevent contamination Sepsis and TPN Serious and crucial complication. Poor technique of catheter care and feed. Mechanical Complications Clotted Catheter Venous Thrombosis Air Embolism Precipitation Air Embolism and TPN This occurs as a result of air entering the central line during set change or catheter insertion. Central Venous Catheters: Mechanical Complications Tracheal/esophageal injury Bleeding Arterial injury Thrombosis of major vein Cardiac arrhythmia Cardiac perforation Endocarditis Pulmonary embolism Nerve injury Thoracic laceration Complications of TPN Central line insertion can lead to: Pneumothorax Brachial plexus injury Arterial puncture Thoracic duct injury Complications of TPN • Nutritional deficiencies such as hypophosphatemia, hypomagnesemia or vitamin and mineral deficiencies may occur. • Thus parentral feeds have to be made nutritionally complete by aseptic admixture in pharmacy. Terminating the Infusion Gradual termination prevents rebound hypoglycemia, especially for diabetic, septic, and stressed patients. TTTTThe endocrine system adjusts to a continuous infusion of dextrose by secreting a certain level of insulin. If the dextrose supply is withdrawn suddenly, the insulin level will not adjust right away, resulting in a relative insulin excess and hypoglycemia. Transitioning to Tube Feeding The TPN infusion should be continued when the tube feeding begins.. If the gut hasn't been used for two or more weeks, enteral feeding tolerance may be compromised. TPN infusion can be decreased in proportion to the increase in tube feeding. • Drug incompatibilities, drug-nutrient interactions and destabilization of lipids can all adversely affect the stability of parenteral nutrition solutions. Medications That Are Compatible Aminophylline Ampicillin Calcium gluconate Cefazolin Cefotaxime Cefoxitin Ceftazidime Ceftriaxone (7% loss in 48 hrs at 20C) Cefuroxime Cimetidine Medications that are incompatible with Parenteral nutrition solutions: 1. 2. 3. 4. 5. 6. Acyclovir Amphotericin B Diazepam Phenytoin Bactrim Metronidazole Therapeutic Incompatibility Antagonistic and synergistic effect Penicillin and cortisone antagonize heparin leading to anticoagulant An increase in amino acid concentration will decrease Theophylline level Anticoagulants Anticoagulants drugs are used in TPN in order to reduce or prevent any tendency toward intravascular or incardiac clotting. Physical Complications Haze detected Particles detected Color changes, Changes from clear to cloudy, Emitting of gas Physical Complications •IN ACIDIC pH • Na DIPHENYL HYDANTOIN •Na PHENOBARBITAL • IN ALKALINE pH •Ca + Na H CO3 • DRAMAMINE + Na H CO3 NITROGLYCERIN HEAT SENSITIVE LIGHT SENSITIVE OXIDATION SENSITIVE HIGHLY ADSORPABLE TO PVC Change in pH can change solubility Antibiotics can remain active in 24 hours at the pH of 6.5, but at pH 3.5 it will be destroy. Potassium Penicillin G buffered at pH 6.0-6.5, when added to dextrose, water or NaCl injection it must also be at buffer 6.0-6.5 to assure activity of antibiotic. • Furosemide X D5W • Verapamil X any (cillin) • Bleomycin X D5W • Enalaprilat X Phenytoin Na • Morphine Sulfate X Acyclovir & Furosemide • Meperidine X Cilastatin Erythromycin + electrolyte Decarbazine + heparin Furosemide + D5W Verapamil + any cillin Na flush Bleomycin + D5W Morphine sulfate + acyclovir + furosemide Morphine + Cilastatin Cyclosporine * plastic bags Polyvinyle chloride leaches 33mg of diethylhexyl phthalate in 48 hours in a glass container Don’t dilute or mix Valium and/or Phenytoin Na because when administered, will make patient bleed. • NOT RECOMMENDED •CEPHALOTHIN SODIUM (KEFLIN) AMPICILLIN SODIUM IN D5W POTENCY DECLINE TO 70% STABILITY OF FROZEN ANTIBIOTIC FOLLOWED BY MICROWAVE RADIATION. Unstable in Plastic Bleomycin Sulfate Carmustine Clonazepan CYCLOSPORIN Diazepan Droperiodel Nitroglycerin Procainamide Vitamin A Warfarin Sodium Insulin MINIMIZATION OF INCOMPATIBILITIES FRESHLY PREPARED FEW ADDITIVES KNOWLEDGEABLE MAKE THEM AWARE ASEPTIC TECHNIQUE KEEP FILE Blood urea nitrogen (BUN) Protein intake is effectively utilized. BUN suggests the nitrogen from the protein source is being converted to urea. Overfed protein, Renal insufficiency. Serum creatinine (Sr.Cr.): Breakdown product of muscle used as a measure of renal function. Transferrin: An iron binding protein present in the blood, involved in the transport of iron Visceral Proteins: Circulating proteins that are synthesized in the liver, such as albumin, pre-albumin and transferrin. www.nyschp.org/the_pharmacist/0998/09 www.ascp.com/public/pubs/tcp/1999/apr/monnut.shtn www.unc.edu/courses/phar 0511/parenterals/text.htm www.ajj.com/services/pblshng/msnj/cconline/0614516 www.pharmj.com www.yahoo.com www.cc.nih.gov www.critcare.1hsc.on www.nyschp.org www.ascp.com www.kumc.edu