* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Inhalational Expect if anthrax
Compartmental models in epidemiology wikipedia , lookup
Public health genomics wikipedia , lookup
Herpes simplex research wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Forensic epidemiology wikipedia , lookup
Antimicrobial resistance wikipedia , lookup
Epidemiology wikipedia , lookup
Antibiotic use in livestock wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
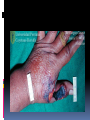
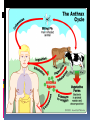
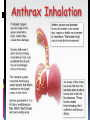
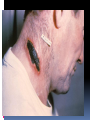
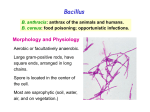
History Caused by Bacillus anthracis Human zoonotic disease Spores found in soil worldwide Primarily disease of herbivorous animals Sheep, goats, cattle Many large documented epizootics Occasional human disease Epidemics have occurred but uncommon Rare in developed world Epidemiology Three forms of natural disease Inhalational Rare (<5%) Most likely encountered in bioterrorism event Cutaneous Most common (95%) Direct contact of spores on skin Gastrointestinal Rare (<5%), never reported in U.S. Ingestion All ages and genders affected Occurs worldwide Endemic areas - Africa, Asia True incidence not known World 20,000-100,000 in 1958 U.S. 235 total reported cases 1955-1994 18 cases inhalational since 1900, last one 1976 Until 2001, last previous case cutaneous 1992 Mortality Inhalational 86-100% (despite treatment) Era of crude intensive supportive care Cutaneous <5% (treated) – 20% (untreated) GI approaches 100% Incubation Period Time from exposure to symptoms Very variable for inhalational 2-43 days reported Theoretically may be up to 100 days Delayed germination of spores Human cases – historical risk factors Agricultural Exposure to livestock Occupational Exposure to wool and hides Woolsorter’s disease = inhalational anthrax Rarely laboratory-acquired Transmission No human-to-human Naturally occurring cases Skin exposure Ingestion Airborne Bioterrorism Aerosol (likely) Small volume powder (possible) Foodborne (unlikely) Transmission Cutaneous Handling hides/skins of infected animals Bites from arthropods (very rare) Handling powdered form in letters, etc. Intentional aerosol release May see some cutaneous if large-scale Microbiology Bacillus anthracis Aerobic, Gram positive rod Long (1-10μm), thin (0.5-2.5μm) Forms inert spores when exposed to O2 Infectious form, hardy Approx 1μm in size Vegetative bacillus state in vivo Result of spore germination Non-infectious, fragile Classification Same family: B. cereus, B. thuringiensis Differentiation from other Bacillus species Non-motile Non β-hemolytic on blood agar Does not ferment salicin Note: Gram positive rods are usually labeled as “contaminants” by micro labs Environmental Survival Spores are hardy Resistant to drying, boiling <10 minutes Survive for years in soil Still viable for decades in perma-frost Favorable soil factors for spore viability High moisture Organic content Alkaline pH High calcium concentration Transmission Inhalational Handling hides/skins of infected animals Microbiology laboratory Intentional aerosol release Small volume powdered form In letters, packages, etc Questionable risk, probably small Transmission Gastrointestinal Ingestion of meat from infected animal Ingestion of intentionally contaminated food Not likely in large scale Spores not as viable in large volumes of water Ingestion from powder-contaminated hands Inhalational of spores on particles >5 m Land in oropharynx Virulence Factors All necessary for full virulence Two plasmids Capsule (plasmid pXO2) Antiphagocytic 3 Exotoxin components (plasmid pXO1) Protective Antigen Edema Factor Lethal Factor Protective Antigen Binds Edema Factor to form Edema Toxin Facilitates entry of Edema Toxin into cells Edema Factor Massive edema by increasing intracellular cAMP Also inhibits neutrophil function Lethal Factor Stimulates macrophage release of TNF-α, IL-1β Initiates cascade of events leading to sepsis Pathogenesis Disease requires entry of spores into body Exposure does not always cause disease Inoculation dose Route of entry Host immune status May depend on pathogen strain characteristics Forms of natural disease Inhalational Cutaneous Gastrointestinal Determined by route of entry Disease occurs wherever spores germinate Pathogenesis. Inhalational Spores on particles 1-5 m Inhaled and deposited into alveoli Estimated LD50 = 2500 – 55,000 spores Dose required for lethal infection in 50% exposed Contained in imperceptibly small volume Inhalational Phagocytosed by alveolar macrophages Migration to mediastinal/hilar lymph nodes Germination into vegetative bacilli Triggered by nutrient-rich environment May be delayed up to 60 days Factors not completely understood Dose, host factors likely play a role Antibiotic exposure may contribute Delayed germination after antibiotic suppression Vegetative bacillus is the virulent phase Active toxin production Hemorrhagic necrotizing mediastinitis Hallmark of inhalational anthrax Manifests as widened mediastinum on CXR Does NOT cause pneumonia Followed by high-grade bacteremia Seeding of multiple organs, including meninges Toxin production Has usually begun by time of early symptoms Stimulates cascade of inflammatory mediators Sepsis Multiorgan failure DIC Eventual cause of death Symptoms mark critical mass of bacterial burden Usually irreversible by this time Clearance of bacteria unhelpful as toxin-mediated Pathogenesis of cutaneous form. Cutaneous Spores in contact with skin Entry through visible cuts or microtrauma Germination in skin Disease begins following germination Toxin production Local edema, erythema, necrosis, lymphocytic infiltrate No abscess or suppurative lesions Eventual eschar formation . In cutaneous anthrax, a malignant pustule develops at the infection site. This pustule is a central area of coagulation necrosis (ulcer) surrounded by a rim of vesicles filled with bloody or clear fluid. A black eschar forms at the ulcer site. Extensive edema surrounds the lesion. The organisms multiply locally and may spread to the bloodstream or other organs (eg, spleen) via the efferent lymphatics. B anthracis remains in the capillaries of invaded organs, and the local and fatal effects of the infection are due, in large part, to the toxins elaborated by B anthracis. Dissemination from the liver, spleen, and kidneys back into the bloodstream may result in bacteremia. Secondary hemorrhagic intestinal foci of anthrax result from B anthracis bacteremia. Cutaneous Systemic disease Can occur, especially if untreated Spores/bacteria carried to regional lymph nodes Lymphangitis/lymphadenitis Same syndrome as inhalational Sepsis, multiorgan failure Pathogenisis GI form Gastrointestinal Spores contact mucosa Oropharynx Ingestion Aerosolized particles >5 m Intestinal mucosa – terminal ileum, cecum Ingestion Larger number of spores required for disease Incubation period 2-5 days Gastrointestinal Spores migrate to lymphatics Submucosal, mucosal lymphatic tissue Mesenteric nodes Germination to vegetative bacilli Toxin production Massive mucosal edema Mucosal ulcers, necrosis Death from perforation or systemic disease Oropharyngeal anthrax Oropharyngeal anthrax is a variant of intestinal anthrax and occurs in the oropharynx after ingestion of meat products contaminated by anthrax. Oropharyngeal anthrax is characterized by throat pain and difficulty in swallowing. The lesion at the site of entry into the oropharynx resembles the cutaneous ulcer. Clinical Features Symptoms depend on form of disease Inhalational Cutaneous Gastrointestinal Inhalational Asymptomatic incubation period Duration 2-43 days, ~10 days in Sverdlovsk Prodromal phase Correlates with germination, toxin production Nonspecific flu-like symptoms Fever, malaise, myalgias Dyspnea, nonproductive cough, mild chest discomfort Duration several hours to ~3 days Can have transient resolution before next phase Fulminant Phase Correlates with high-grade bacteremia/toxemia Critically Ill Fever, diaphoresis Respiratory distress/failure, cyanosis Septic shock, multiorgan failure, DIC 50% develop hemorrhagic meningitis Headache, meningismus, delirium, coma May be most prominent finding Usually progresses to death in <36 hrs Mean time from symptom onset to death ~3 days Laboratory Findings Gram positive bacilli in direct blood smear Electrolyte imbalances common Radiographic Findings Widened mediastinum Minimal or no infiltrates Can appear during prodrome phase Cutaneous Most common areas of exposure Hands/arms Neck/head Incubation period 3-5 days typical 12 days maximum Cutaneous – progression of painless lesions Papule – pruritic Vesicle/bulla Ulcer – contains organisms, sig. edema Eschar – black, rarely scars Systemic disease may develop Lymphangitis and lymphadenopathy If untreated, can progress to sepsis, death Gastrointestinal Oropharyngeal Oral or esophageal ulcer Regional lymphadenopathy Edema, ascites Sepsis Abdominal Early symptoms - nausea, vomiting, malaise Late - hematochezia, acute abdomen, ascites Diagnosis. Early diagnosis is difficult Non specific symptoms Initially mild No readily available rapid specific tests Presumptive diagnosis History of possible exposure Typical signs & symptoms Rapidly progressing nonspecific illness Widened mediastinum on CXR Large Gram+ bacilli from specimens Can be seen on Gram stain if hi-grade bacteremia Appropriate colonial morphology Necrotizing mediastinitis, meningitis at autopsy Definitive diagnosis Direct culture on standard blood agar Gold standard, widely available Alert lab to work up Gram + bacilli if found 6-24 hours to grow Sensitivity depends on severity, prior antibiotic Blood, fluid from skin lesions, pleural fluid, CSF, ascites Sputum unlikely to be helpful (not a pneumonia) Very high specificity if non-motile, non-hemolytic Requires biochemical tests for >99% confirmation Available at Reference laboratories Other diagnostic tests Anthraxin skin test Chemical extract of nonpathogenic B. anthracis Subdermal injection 82% sensitivity for cases within 3 days symptoms 99% sensitivity 4 weeks after symptom onset Testing for exposure Nasal swabs Can detect spores prior to illness Currently used only as epidemiologic tool Decision based on exposure risk May be useful for antibiotic sensitivity in exposed Culture on standard media Swabs of nares and facial skin Serologies May be useful from epidemiologic standpoint Investigational – only available at CDC Differential diagnostics Inhalational Influenza Pneumonia Community-acquired Atypical Pneumonic tularemia Pneumonic plague Mediastinitis Bacterial meningitis Thoracic aortic aneurysm Expect if anthrax Flu rapid diagnostic – More severe in young pts No infiltrate No prior surgery Bloody CSF with GPBs Fever Cutaneous Spider bite Ecthyma gangrenosum Pyoderma gangrenosum Ulceroglandular tularemia Mycobacterial ulcer Cellulitis Expect if anthrax fever no response to 3º cephs painless, black eschar +/- lymphadenopathy usually sig. local edema Gastrointestinal Gastroenteritis Typhoid Peritonitis Perforated ulcer Bowel obstruction Expect if anthrax Critically ill Acute abdomen Bloody diarrhea Fever Treatment. Hospitalization IV antibiotics Empiric until sensitivities are known Intensive supportive care Electrolyte and acid-base imbalances Mechanical ventilation Hemodynamic support Antibiotic selection Naturally occurring strains Rare penicillin resistance, but inducible βlactamase Penicillins, aminoglycosides, tetracyclines, erythromycin, chloramphenicol have been effective Ciprofloxacin very effective in vitro, animal studies Other fluoroquinolones probably effective Engineered strains Known penicillin, tetracycline resistance Highly resistant strains = mortality of untreated Empiric Therapy Until susceptibility patterns known Adults Ciprofloxacin 400 mg IV q12° OR Doxycycline 100mg IV q12° AND (for inhalational) One or two other antibiotics Pregnant women Same as other adults Weigh small risks (fetal arthropathy) vs benefit Immunosuppressed same as other adults Susceptibility testing should be done Narrow antibiotic if possible Must be cautious Multiple strains with engineered resistance to different antibiotics may be coinfecting Watch for clinical response after switching antibiotic Antibiotic therapy Duration 60 days Risk of delayed spore germination Vaccine availability Could reduce to 30-45 days therapy Stop antibiotics after 3rd vaccine dose Switch to oral Clinical improvement Patient able to tolerate oral medications Other therapies Passive immunization Anthrax immunoglobulin from horse serum Risk of serum sickness Antitoxin Mutated Protective Antigen Blocks cell entry of toxin Still immunogenic, could be an alternative vaccine Animal models promising Postexposure Prophylaxis Who should receive PEP? Anyone exposed to anthrax Not for contacts of cases, unless also exposed Empiric antibiotic therapy Vaccination Avoid unnecessary antibiotic usage Potential shortages of those who need them Potential adverse effects Hypersensitivity Neurological side effects, especially elderly Bone/cartilage disease in children Oral contraceptive failure Future antibiotic resistance Individual’s own flora Community resistance patterns Post exposure prophylaxis. Antibiotic therapy Treat ASAP Prompt therapy can improve survival Continue for 60 days 30-45 days if vaccine administered Antibiotic therapy Same regimen as active treatment Substituting oral equivalent for IV Ciprofloxacin 500 mg po bid empirically Alternatives Doxycycline 100 mg po bid Amoxicillin 500 mg po tid Prevention. Vaccine Anthrax Vaccine Adsorpbed (AVA) Supply Limited, controlled by CDC Production problems Single producer – Bioport, Michigan Failed FDA standards None produced since 1998 Vaccine Inactivated, cell-free filtrate Adsorbed onto Al(OH)3 Protective Antigen Immunogenic component Necessary but not sufficient Vaccine Administration Dose schedule 0, 2 & 4 wks; 6, 12 & 18 months initial series Annual booster 0.5 ml SQ Vaccine Adverse Effects >1.6 million doses given to military by 4/2000 No deaths <10% moderate/severe local reactions Erythema, edema <1% systemic reactions Fever, malaise Infection control. No person to person transmission Standard Precautions Laboratory safety Biosafety Level (BSL) 2 Precautions Decontamination. Skin, clothing Thorough washing with soap and water Avoid bleach on skin Instruments for invasive procedures Sterilize, e.g. 5% hypochlorite solution Sporicidal agents Sodium or calcium hypochlorite (bleach)