* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Adverse drug reaction (ADR)
Survey
Document related concepts
Transcript
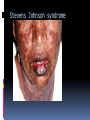
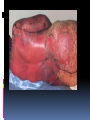
CLASSIFICATION, MECHANISMS, CLINICAL MANIFESTATIONS INTRODUCTION TO ADVERSE DRUG REACTIONS What is PHARMACOVIGILANCE? Science and activities relating to the detection, assessment, understanding & prevention of adverse effects or any other possible drug-related problems. Etymology: pharmakon (Greek), “drug” vigilare (Latin), “to keep awake or alert, to keep watch” What is Pharmacovigilance? “is the science of collecting, monitoring, researching, assessing and evaluating information from healthcare providers and patients on the adverse effects of medications, biological products, herbal and traditional medicines with a view to identifying new information about hazards associated with medicines and preventing harm to patients” Particularly concerned with adverse drug reactions; also medication errors & drug interactions Objectives To enumerate and give examples of the different types of adverse drug reactions To explain mechanisms of adverse drug reactions To describe strategies to prevent or minimize their occurrence DEFINITIONS Adverse drug reaction (ADR): response to a drug that is noxious & unintended, and which occurs at doses normally used in man for the prophylaxis, diagnosis, or the therapy of disease, or for the modification of physiological function. (WHO) Adverse drug event (ADE): an untoward and unexpected experience by a pt following the use of a medicinal product but does not necessarily have a causal relationship with the treatment. ADE versus ADR ADE does not confer a definitive causality relationship between the drug and the event but just a mere suspicion. ADR on the other hand, defines a more certain or probable relationship, the mechanisms of which might be explained by some pharmacological actions or in some cases, cannot yet be conventionally explained Difficulties with ADR’s ADR’s masquerade as other diseases Drug effects are the consequence of complex interactions between the drugs, the patient and the illness and external factors can also modify drug response. ADR’s: drug-induced illness Incidence of ADR’s (US data) 30% of hospitalized patients will have one or more ADRs while in the hospital 3% with severe reactions 0.3% will die 5% of admissions to the medical service are caused by ADRs developed on an out- patient basis extremes of age women (60%) > men (40%) patients with past history of reactions to medications IMPACT OF AE’s/ADR’s on PUBLIC HEALTH ADR’s are the 4th- to 6th largest cause of mortality in the USA. 16% of hospital admissions in the UK is due to adverse drug reactions 9 Economic consequences Some countries spend up to 15-20% of their hospital budget dealing with drug complications Direct cost of managing drug-related morbidity and mortality in the ambulatory setting : approx USD $ 76.6 B per year (1995) 10 Significance of ADR: Vary in significance according to their nature and circumstances: significant or insignificant apparent or hidden severe or mild acute or chronic immediate or delayed Factors Affecting ADR’s (manifestation/severity) •Patient-related factors Age Sex Genetic influences Concurrent diseases (renal, liver, cardiac) Previous adverse drug reactions Compliance with dosing regimen Total number of medications Misc. (diet, smoking, environmental exposure) Factors Affecting Adverse Drug Reactions : • Drug-related factors: Dose Duration Inherent toxicity of the agent Pharmacodynamic properties Pharmacokinetic properties Types of Adverse Drug Reactions : • Type A ( Augmented ) • Type B ( Bizarre ) • Type C ( Continuous ) • Type D ( Delayed ) • Type E ( Ending of Use ) • Type F ( Failure of Treatment ) TYPE A ( AUGMENTED ) An effect that is higher in intensity or magnitude than the expected pharmacological effect. Can occur either as an extension effect or part of the therapeutic effect or can occur as not part of the intended therapeutic effect (as a side-effect). It has usually a dose-dependent mechanism. TYPE A ( AUGMENTED ) Augmented extension effect – an ADR resulting from the intended or therapeutic effect of the drug. - It is expected, and can be managed with dose reduction or shifting to another class of drugs. Augmented side effect –an ADR that is not the intended or therapeutic effect; TYPE A ( AUGMENTED ) • Extension Effect: • predictable, dose-related responses arising from an extension of therapeutic effect E.g. Benzodiazepine - sedation Insulin - hypoglycemia Heparin - spontaneous bleeding Beta-blocker - bradycardia TYPE A ( AUGMENTED ) • Side Effect: • predictable, dose-dependent reactions unrelated to the goal of therapy E.g. NSAID’s: Upper GI bleed Beta-agonists (salbutamol): Tremors, tachycardia TYPE B (BIZARRE) Can be likened to a hypersensitivity or idiosyncratic reactions. Often unpredictable. No conventional tests to demonstrate which pts might experience this ADR in order to theoretically prevent its occurrence. Not dose dependent to the medicine used TYPE B (BIZARRE) Some can occur immediately such as penicillin hypersensitivity & acute anaphylactic reactions. But some can occur at a much later time, even months later, such as Steven-Johnson due to anti-zeizure medications such as phenytoin, carbamazepine, Phenobarbital, or sulfa-containing drug Not all cases can be explained by known pharmacological actions of the suspected drug. TYPE B (BIZARRE) • No formal dose-response curve; very small doses of the drug may elicit the reaction once allergy is established • Reaction disappears on discontinuation of the drug • Illness is often recognizable as an immunological reaction • No relation to the usual pharmacological effects of the drug Stevens–Johnson syndrome (SJS) A life-threatening condition affecting the skin in which cell death causes the epidermis to separate from the dermis An unusual, severe reaction characterized by blistering and sloughing of the mucous membranes; the visceral organs may also be involved, and the condition can be fatal. Stevens Johnson syndrome TYPE B (BIZARRE) SYNDROMES of TYPE B ADR’s in CLINICAL PRACTICE • Connective tissue disease: drug-induced lupus • Blood disorders: agranulocytosis • Respiratory disorders • Fever • Rashes and skin lesions IDIOSYNCRACY • genetically determined abnormal response to a drug • although dose-dependent, such reactions are unpredictable in most instances • cannot be attributed to drug allergy E.g. Aspirin/sulphonamides - hemolytic anemia due to erythrocyte G-6PD deficiency Drugs and Chemicals Likely to Induce Hemolytic Anemia in G6PD Deficiency Dapsone Furazolidone Glyburide Methylene Blue Nalidixic Acid Naphthalene Nitrofurantoin Primaquine Spiramycin Sulfacetamide Sulfamethoxazole Sulfanilamide Toluidine Blue Trinitrotoluene C-CONTINUOUS This ADR happens after a prolonged use of a drug even at normal dosage. Related to DOSE & duration of treatment It affects organ systems. Long Term use of Steroids: Cushing’s syndrome INH for TB: hepatitis, neuropathy. D-DELAYED Seen when a drug used at some earlier time has some adverse effects observed much later on, such as affecting the next generation. Diethylstilbestrol taken by women can cause vaginal & other reproductive organ damage in female offspring (vaginal CA). 1960’s thalidomide used by pregnant women invariably leads to the development of congenital malformations (phocomelia). D-DELAYED • Carcinogenesis • Teratogenecity • Immunotoxicity E-ENDING OF USE (withdrawal syndromes) • When a drug that was used on long term is suddenly stopped, the pt suffers a form of withdrawal reaction. • These drugs exhibit tolerance phenomenon or have some dependency potential. • Examples are: rebound hypertension following sudden cessation of clonidine, adrenal insufficiency after stopping prolonged steroid use, seizures from acutely ceasing anticonvulsants, and uncomfortable withdrawal syndromes from benzodiazepines and narcotics. Hypothalamo-Pituitary Regulation of Cortisol Secretion Neural Stimuli Neural Stimuli Corticotropin Releasing Center Hypothalamus CRF Anterior Pituitary Plasma Cortisol Concentration ACTH ACTH Plasma Cortisol Concentration Adrenal Cortex Type F- FAILURE OF TREATMENT • Substandard drug • No actual drug inside • Toxic excipients • Tolerance effects • Antimicrobial resistance Type F- FAILURE OF TREATMENT • A new form of ADR recently recognized as a public health threat. • ADR monitoring systems can pick up unusual and unexpected drug inefficacy and can detect possible fake, or substandard quality medicines. • Once detected, info can be useful to the drug regulators, concerned industry & to the institutions that use these drugs. • Patients’ lives & the public health is the ultimate beneficiary. • Example: Oxytocin case Classifying ADR’s accdg to seriousness and severity ADR symptoms can be described as mild, moderate, severe. These are descriptive terms of the intensity of a particular sign or symptom. Serious, on the other hand defines the urgency and the impending critical threat to the life of the patient or to an organ-system. Serious versus severe The term “severe” is often used to describe the intensity (severity) of a specific event (as in mild, moderate or severe myocardial infarction; the event itself, however, may be of relatively minor medical significance e.g. a severe headache may not be serious but a mild stroke resulting in disability is serious Explaining Risks to Patients When doctors explain drug risks to patients, he will use lay terms. So how common is common ? Common or Frequent is somewhere between 1 in 100 (1%) and 1 in 10 (10%). Uncommon or infrequent : between 1 in 1,000 (0.1%) and 1 in 100 (1%) Rare is between 1 in 10,000 (0.01%) and 1 in 1,000 (0.1%) Interventions If and when a prescriber suspect an ADR, he is in a critical position to intervene. The possible interventions are as follows: Stop the drug or Stop all of the drugs (dechallenge) Change to another medication Maintain the use of the drug. Modify the dosages Use another medication to modify the ADR Interventions Identify possible drug – drug interactions Advise the pt whether to stop or to continue the use of that medications depending on risk – benefit considerations. Report the incident (the collection point for ADE is the Bureau of Food and Drugs). Do research. Establish causality through a system of analysis and assessment. Re-instituting the stopped medications should not be done just to re-affirm a suspicion of ADR as this might be dangerous (re-challenge). The most important thing to remember is always monitor the patient for the expectant good effects of the medicines but also look for the possible or potential negative effects. Reporting While it is not mandatory for health professionals to report observed ADR cases, in the interest of public health, it is suggested that all doctors, nurses and pharmacists report suspected and serious, lifethreatening ADRs. These can be the known and the unexpected, to the Food and Drugs Administration of the Department of Health using a standard form. Reporting While the incidence may be rare in your perspective, if there are similar cases that arose in other areas and are similarly reported, a trend may be established for further investigations or for signaling. An early warning can save lives. . Purpose of Safety Reports To learn from experience (of others) Reporting in itself does not improve safety. Collecting data contributes little to pt safety advancement It is the response to reports that leads to change. 43 Purpose of Safety Reports Within a health-care institution, reporting of a serious event or serious “near-miss” should trigger an indepth investigation to identify underlying systems failures and lead to efforts to redesign the systems to prevent recurrence For national/international systems, to recognize trends and patterns in occurrence of AE’s: generating safety signals 44 Simple Rules to Guide the Prescriber Use the lowest dose possible and titrate dose accordingly (individualized management). Don’t be afraid to use a medicine when it is really indicated just because of the known risk. Use as few medicines as possible, because the incidence of ADRs increases with the number of drugs Following the decision to use medicines, educate your pt on what to expect and what to do in case of some untoward events. Simple Rules to Guide the Prescriber The prescriber should monitor both the expected good and the unexpected bad effects. Even the over-the-counter medicines that do not require a prescription may cause adverse reactions. Some doctors are afraid to modify the prescription of their peers who are co-managing their patient. In the interest of patient safety, do not be afraid to consult your peers if you think that the drugs they prescribed are suspected to be cause of the ADRs. Spontaneous Reporting • identification of rare adverse effects • monitor newly introduced drugs • hypothesis generating and raising signals or flags • support the regulatory policies • contribute to improvement in health policies and practices • reports are not admission of causality