* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download HBV Training Workshop
African trypanosomiasis wikipedia , lookup
Microbicides for sexually transmitted diseases wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Leptospirosis wikipedia , lookup
Chagas disease wikipedia , lookup
Neonatal infection wikipedia , lookup
Antiviral drug wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Oesophagostomum wikipedia , lookup
Schistosomiasis wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
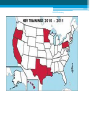
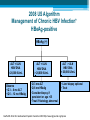
HBV Training Workshop Alan Franciscus Editor-in-Chief HBV Advocate / HCV Advocate WWW.HBVAdvocate.org WWW.HBVAdvocate.org www.HBVAdvocate.org WWW.HBVAdvocate.org www.hcvadvocate.org www.hepatitistattoos.org WWW.HBVAdvocate.org WWW.HBVAdvocate.org The Liver • About 3 lbs (men) – size of a football • Blood organ • Chemical factory > 500 chemical functions • Metabolizes sugar and fat • Stores some vitamins and minerals WWW.HBVAdvocate.org The Liver • Filters and Detoxifies ▫ Breathed in the air, absorbed through the skin & by mouth • Makes proteins to help the blood clot • The liver can regenerate • Non-complaining organ WWW.HBVAdvocate.org Keep the Liver Healthy • Be careful with alcohol and drugs ▫ Healthy People: No more than 2 alcoholic drinks a day – men; no more than 1 alcoholic drink a day for women People with HBV – avoid alcohol • Get vaccinated against HAV & HBV • Eat a healthy, balanced diet HBV Transmission & Prevention WWW.HBVAdvocate.org HBV is 50 to 100 times more infectious than HIV WWW.HBVAdvocate.org HBV Worldwide • 2 billion people worldwide are infected with HBV ▫ An estimated 400-800 million people have chronic hepatitis B (CHB)2,3 • Complications from HBV are the 10th leading cause of death worldwide § Immigration From Endemic Areas Impacts CHB Prevalence In The United States • Majority of immigrants have never been vaccinated against HBV • CDC estimates 450,000 immigrants admitted to the USA between 19942003 were infected with hepatitis B WWW.HBVAdvocate.org Estimates - HBV Statistics - U.S. • 43,000 new or acute infections • 2-3 million – chronic infections - ~65% unaware • 3,000-4,000 deaths a year ▫ 70% of deaths are from liver cancer • ~1 of 8 Vietnamese Americans • ~1 of 10 Chinese Americans • ~1 of 12 Korean Americans Asian Population in the United States, 2000 = ~12 million4,5 • The Asian American community is projected to grow to 33.4 million people (or 8% of the total US population) by 2050 ▫ 68.9% of Asian Americans living in US are foreign-born ▫ Asian Americans are 2.7 times more likely to develop hepatocellular carcinoma (HCC) and 2.4 times more likely to die from HCC than their white counterparts Korean Chinese 2,734,841 1,228,427 Taiwanese Asian Indian Hmong 1,899,599 144,795 186,310 Laotian 198,203 Vietnamese 1,223,736 Filipino 2,364,815 Samoan5 133,281 WWW.HBVAdvocate.org HBV Transmission: Concentrations of HBV in Body Fluids HIGH MODERATE LOW/ NOT DETECTED Blood Serum Wounds Semen Vaginal fluid Saliva Urine Feces Sweat Tears Breast milk WWW.HBVAdvocate.org HBV Transmission (more) • Blood borne – can live outside the body for at least 7 days • Highly infectious in semen and vaginal secretions • Sharing needles and works to inject drugs • Needle stick accidents, Healthcare exposure • Household - sharing personal items • Horizontal – childhood – biting, scratching • Vertical-Mother-to-Child transmission at birth § Epidemiologic Characteristics of Patients With Acute Hepatitis B US, 2007* • More than 70% of acute infections reported in 2007 were attributed to ▫ sexual activity ▫ injection drug use (IDU) • Sexual activity accounts for most HBV transmission in the US Cases Reported with Risk Factor Data Characteristic Had > 1 sex partner Homosexual activity (male) Sexual contact with CHB patient Injection-drug use Surgery Household contact of CHB patient Percutaneous injury (i.e. needlestick) Medical employee with blood contact Hemodialysis, blood transfusion Unknown *Values Daniel D. Acute Viral Hepatitis in US, 2007. MMWR 2009;58(No.SS-3). total > 100% because multiple risk factors could be reported for a single case %* 38.3 10.5 6.2 15 11.7 2.3 4.3 0.6 0.2, 0.6 58 § CDC Recommends Screening Adults at High Risk for HBV Infection Populations Sexual exposure • • • • Percutaneous or mucosal exposure to blood • • • • Increased HBsAg Prevalence • Persons born in regions with high or intermediate prevalence of HBV infection (HBsAg prevalence ≥2%) • U.S.-born persons not vaccinated as infants whose parents were born In regions with high prevalence of HBV infection (HBsAg prevalence ≥8%) Increased Risk of Medical Consequences • • • • Sex partners of HBsAg-positive persons Sexually active persons not in a long-term, mutually monogamous relationship Persons seeking evaluation or treatment for a sexually transmitted disease Men who have sex with men Current or recent IDU Household contacts of HBsAg-positive persons Residents and staff of facilities for developmentally disabled persons Healthcare and public safety workers with risk for exposure to blood or bloodcontaminated body fluids • Persons with end-stage renal disease HIV+ persons International travelers to regions endemic with HBV infection (prevalence of ≥2%) Persons with immunosuppressive therapy Persons with elevated ALT or AST of unknown etiology Weinbaum CM, et al. MMWR Recomm Rep. 2008;57(RR-8):1-20 16 WWW.HBVAdvocate.org Screen for HBV: US persons not vaccinated as infants whose parents were born in regions with HBV prevalence ≥8% (in red) Screen for HBV: Persons born in regions with HBV prevalence ≥ 2% (in red) > 2% - Intermediate to high risk (should be screened with or w/o additional risk factors) <2% - Low (not required to screen without additional risk factors) Centers for Disease Control and Prevention. MMWR. 2006;55(RR16). Accessed online October 16, 2007. WWW.HBVAdvocate.org HBV Prevention: CDC Strategy to Eliminate HBV • Vaccination at birth • Screening of all pregnant women • Vaccination of all previously unvaccinated children and adolescents • Vaccination of previously unvaccinated adults at risk for HBV infection WWW.HBVAdvocate.org HBV Prevention: Recommended for Vaccination • Sexual exposure: ▫ Sexual contacts of HBV positive persons ▫ People who are sexually active with more than one sexual partner w/i the last 6 months ▫ People seeking STD services ▫ Men who have sex with men WWW.HBVAdvocate.org HBV Prevention: Recommended for Vaccination • Blood/mucous exposure: ▫ Current or recent IDU ▫ Household contact ▫ Residents and staff of facilities for developmentally disabled Americans ▫ Healthcare and public safety workers who may come into contact with blood/bodily fluids ▫ People with kidney disease – hemodialysis WWW.HBVAdvocate.org HBV Prevention: Recommended for Vaccination • Others: ▫ International travelers who travel to countries that have higher or intermediate levels of HBV ▫ Persons with chronic liver disease ▫ Persons with HIV ▫ All persons seeking protection from HBV infection WWW.HBVAdvocate.org HBV Prevention • HBV Vaccination – 3 dose series (Twinrix HAV & HBV) • Not all respond / not all countries have vaccines • Safer sex • Standard safety/universal precautions • Do not share needles or works to inject drugs ▫ Needle Exchange! • Do not share personal items (razors, toothbrushes) WWW.HBVAdvocate.org More Prevention — Mother to Child • Every pregnant woman should be screened for HBV • 1 in 5 not screened • Ok to breast feed infants born to HBV infected mothers • Up to 90% of infants born to mothers with chronic HBV will become chronic unless: ▫ Infant is vaccinated and given immune globulin within 12 hours of birth – reduces chronic rate to ~10% • Treating pregnant women with HBV medications – no clear recommendations Diagnosing HBV WWW.HBVAdvocate.org •HBV discovered in 1967 by Dr. Blumberg and colleagues •Dr. Blumberg awarded Nobel Prize in Medicine in 1976 WWW.HBVAdvocate.org WWW.HBVAdvocate.org Keep it Simple! • HBV Antibodies (proteins made by body) • HBV Antigens (HBV viral proteins) • No HBV surface antibody and no surface antigen – susceptible – vaccinate • HBV surface antibody – protected • HBV DNA (viral load) > 6 months – chronic • Gray areas???? WWW.HBVAdvocate.org HBV DNA — Viral Load • Expressed in ‘International Units’ – IU/mL ▫ Previously reported in copies — IU/mL = 5 - 6 copies ▫ Range: 10 to millions or billions • Used to: ▫ Confirm active infection ▫ Monitoring ▫ Treatment of chronic HBV WWW.HBVAdvocate.org HBV Genotype • 8 different genotypes – A thru H ▫ Not routinely performed ▫ Genotypes A & B – pegylated interferon ▫ Genotype C – increased risk for disease progression and liver cancer WWW.HBVAdvocate.org Laboratory Tests • Liver tests (ALT/AST): Healthy ALT considered to be <19 for women and <30 for men • CBC, platelets, prothrombin time • Liver biopsy • AFP – liver cancer • Ultrasound/MRI/CTscan – screen for liver cancer (HCC) Chronic HBV: Symptoms, Progression and Management WWW.HBVAdvocate.org HBV is the second most important carcinogen after tobacco WWW.HBVAdvocate.org Symptoms Acute ▫ ▫ ▫ ▫ ▫ ▫ ▫ ▫ Fever Fatigue Loss of appetite Nausea Vomiting Dark urine Clay-colored stools Jaundice ◦ And more…… • Children typically exhibit no symptoms Chronic • • • • • Fatigue Fever Abdominal pain Muscle & joint pain Nausea • And more……….. • Chronic – most people have no symptoms WWW.HBVAdvocate.org Chronic Infection • ~90% of Infants born to HBV-infected Mothers ▫ Intervention decreases chronic rate to ~10% ▫ 25 to 50% of children aged 1-5 years • ~5-6% of Adults WWW.HBVAdvocate.org Disease Progression • 3,000 – 4,000 deaths a year • ~15 - 25% develop serious disease progression including cirrhosis, liver failure or liver cancer ▫ Usually after 20 to 30 years • Risk factors for disease progression ▫ Host – male gender, advanced age, alcohol use & cigarette smoking ▫ Other factors – persistent high viral load, coinfection (HIV or HDV), immunosuppression, HBV genotype C, HBV mutations, severity and frequency of ALT elevations ▫ Family history of liver cancer increases risk by 2 fold WWW.HBVAdvocate.org WWW.HBVAdvocate.org Managing Chronic HBV • Liver biopsy • Regular office visits and tests ▫ Medical provider will set up a regular schedule of visits and tests Common tests: ALT levels, HBV DNA (viral load), HBV viral markers, HBV genotype ▫ Screen every 6 to 12 months for liver cancer (AFP / Ultrasound) • Avoid alcohol, tobacco and anything that can harm the liver WWW.HBVAdvocate.org Managing HBV - continued • Exercise • Support • Healthy diet: ▫ www.mypyramid.gov Treatment of Chronic HBV WWW.HBVAdvocate.org 39 Goals of Treatment for Chronic HBV Overall Goals: • Prevent complications of chronic HBV: ▫ Cirrhosis, hepatocellular carcinoma (HCC = liver cancer), death • Suppression of HBV Markers of Treatment Response: • Decrease serum HBV DNA (viral load) to low or undetectable levels • Improve liver histology • Lowering or normalization of ALT levels Lok ASF. Hepatology. 2004;39:857-861. Keeffe EB. Clin Gastroenterol Hepatol 2006;4:936-962. WWW.HBVAdvocate.org Keep It Simple! • Treat: ▫ Elevated ALT ▫ Elevated HBV DNA ▫ Treatment, however, is a complicated process that takes into account many factors – see next series of slides…. 2008 US Algorithm Management of Chronic HBV Infection* HBeAg-positive HBeAg (+) ALT < ULN HBV DNA < 20,000 IU/mL Observe • Q 3 – 6 mo ALT • Q 6 – 12 mo HBeAg ALT < ULN HBV DNA > 20,000 IU/mL • Q 3 mo ALT • Q 6 mo HBeAg • Consider biopsy if persistent or age >35 • Treat if histology abnormal ALT > ULN HBV DNA > 20,000 IU/mL • Liver biopsy optional • Treat Keeffe EB. Et al Clin Gastroenterol Hepatol. December 2008 http://www.cghjournal.org/inpress 2008 US Algorithm Management of Chronic HBV Infection HBeAg-negative HBeAg (-) ALT < ULN; HBV DNA < 2,000 IU/mL • Q 3 mo ALT x 3, then Q 6 – 12 mo if ALT still <1 x ULN ALT < ULN; HBV DNA > 2,000IU/mL • Q 3 mo ALT & HBV DNA • Consider biopsy if persistent DNA elevation or age >35 • Treat if histology abnormal ALT > ULN; HBV DNA > 2,000 IU/mL • Liver biopsy optional • Treat Keeffe EB. Et al Clin Gastroenterol Hepatol. December 2008 http://www.cghjournal.org/inpress 2008 US Algorithm Management of Chronic HBV Infection Patients with Cirrhosis Compensated HBV DNA <2,000 IU/mL HBV DNA ≥2,000 IU/mL Decompensated Detectable HBV DNA Treat Observe or Treat Undetectable HBV DNA Observe Treat Wait List for Transplant Keeffe EB. Clin clinical Gstroenterol Hepatol.associated 2006;4:936-962. *Significant consequences with LAM resistance in this population Keeffe EB. Et al Clin Gastroenterol Hepatol. December 2008 http://www.cghjournal.org/inpress WWW.HBVAdvocate.org Approved HBV Medications Generic Name Brand Name Manufacturer Date Approved Interferon alfa-2b INTRON® A Merck/Schering 1991 Lamivudine EPIVIR-HBV® GlaxoSmithKline 1998 Adefovir dipivoxil HEPSERA ™ Gilead Sciences 2002 Entecavir BARACLUDE ™ Bristol-Myers Squibb 2005 Peginterferon alfa-2a PEGASYS® Genentech/Roche 2005 Telbivudine TYZEKA ™ Idenix/Novartis 2006 Tenofovir VIREAD ™ Gilead Sciences 2008 WWW.HBVAdvocate.org HBV Drugs and Resistance *Entecavir, peginterferon alfa-2a and tenofovir recommended as first line of treatment WWW.HBVAdvocate.org HBV Treatment Side Effects • Direct antivirals: ▫ Minimal side effects – fatigue, stomach, diarrhea, muscle weakness and pain ▫ Need to monitor renal function for dosing • Pegylated interferon: ▫ More severe type of side effects – moderate to severe fatigue, depression, anxiety, gastro, body aches and pains, insomnia, etc. WWW.HBVAdvocate.org Chronic HBV Medications • 100% medications; 100% of the time ▫ Resistance ▫ Ask about adherence ▫ Potentially produce a flare –up–small % fulminate • Need to take fasting (2hrs prior to or 2hrs after a meal): BARACLUDE/entecavir • Monitoring during treatment WWW.HBVAdvocate.org Pregnancy Drug Categories Drug Category Indication Tenofovir (Viread) B HBV and HIV Telbivudine (Tyzeka) B HBV Interferon (Intron A) C HCV and HBV Pegylated interferon alfa-2a (Pegasys) C HCV and HBV Pegylated interferon alfa-2b (PegIntron) C HCV Entecavir (Baraclude) C HBV Adefovir (Hepsera) C HBV Lamivudine (Epivir-HB) C HBV and HIV WWW.HBVAdvocate.org Complementary and Alternative Therapies • Herbs have the potential to cause damage and interact with other herbs and medications ▫ Inform your medical provider ▫ Use a reputable herbalist ▫ Acupuncture & Acupressure ▫ Traditional Chinese Medicine Meditation, qi qong, tai chi, massage, acupuncture, acupressure, moxibustion. WWW.HBVAdvocate.org Patient Assistance Programs • Needymeds.org • Partnership for Prescription Assistance • • • • • Gilead Pegasys GSK BMS Idenix/Norvartis WWW.HBVAdvocate.org Recommended Websites • HBV Advocate: www.HBVAdvocate.org • CDC – Viral Hepatitis: www.cdc.gov/hepatitis/ • Hepatitis B Foundation: www.hbf.org • www.hivandhepatitis.com