* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Maraviroc (Selzentry)
Survey
Document related concepts
Transcript
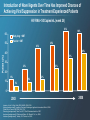
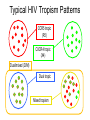
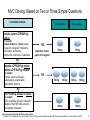
CCR5 Antagonist Maraviroc (Selzentry) It’s new, it’s novel, it’s niche? Blake Max, PharmD RMR CORE Center Clinical Assistant Professor, UIC College of Pharmacy Objectives • Describe the mechanism of action of CCR5 antagonist • Understand the role and importance of the Trofile assay in relation to CCR5 antagonist use • Describe the significant clinical trials in tx-naïve and experienced patients • Infer optimal CCR5 antagonist dose when administered with other medications or patient populations Introduction • 5 Classes of Antiretroviral Agents - NRTIs - NNRTIs - PIs - Integrase Inhibitors - Entry Inhibitors • August 2007 FDA approved maraviroc, a CCR5 coreceptor antagonist used in combination with other antiretroviral agents for treatment in adults (>16 y/o) infected with CCR5-tropic HIV. Introduction of Novel Agents Over Time Has Improved Chances of Achieving Viral Suppression in Treatment-Experienced Patients HIV RNA <50 Copies/mL (week 24) 63% 60 50 Study drug + OBT Placebo + OBT 45% 45% 40% 40 Patients (%) 60% 33% 30 23% 23% 20 12% 10 9% 12% 5% 0 2003 Lazzarin A et al. N Engl J Med. 2003;348(22):2186-2195. Aptivus [package insert]. Ingelheim, Germany: Boehringer Ingelheim International GmbH; 2006. Clotet B et al. Lancet. 2007;369:1169-1178. Selzentry [package insert]. New York, NY: Pfizer Pharmaceuticals Inc; 2007. Isentress [package insert]. Whitehouse Station, NJ: Merck & Co., Inc; 2008. Intelence [package insert]. Yardley, PA: Tibotec, Inc; 2008. 2008 Novel Mechanism of Action • HIV Entry Inhibitors Enfuvirtide- synthetic peptide that mimics amino acids of HIV transmembrane protein (gp41), which is critical for viral/CD4 cell membrane fusion. Maraviroc- HIV surface protein (gp120) binds to CD4 surface protein, acting as an anchor, then additional interaction with CD4 surface co-receptor allows for viral entry. HIV uses two co-receptors, CCR5 and CXCR4, maraviroc is a CCR5 antagonist. Vicriviroc- Not FDA approved, in clinical trials, same MOA as maraviroc. CD4 Co-receptors: CCR5 and CXCR4 Binding, and Fusion Targets for Inhibition CD4 Attachment CD4 attachment inhibitors gp41 Co-receptor Binding CCR5 antagonists Virus–Cell Fusion Fusion inhibitors gp120 V3 loop CD4 Cell membrane CCR5 or CXCR4 Adapted from Moore JP, et al. Proc Natl Acad Sci USA 2003;100:10598–602 Approx. 50% of HIV Tropism is CCR5 in Treatment Experienced Patients R5 Tropism Result is Strongly Associated with CD4+ Cell Count Categories in Treatment Experienced Patients South African Cohort (~94% clade C) MOTIVATE 1 and 2 (~96% clade B) R5 D/M X4 100 80 60 60 40 0 N= 22 40 R5* D/M 20 20 X4 25 68 56 119 11 Current CD4+ cell count (cells/mm3) 0 N= Screening CD4+ Category (cells/mm3) * P<0.0001 for comparison of median screening CD4+ cell counts as a continuous variable Eng SM et al. 9th Intl Cong. on Drug Ther in HIV Infect.2008. Poster P198 Clax P et al. EI Wrkshp 2007. Abs 5 Percent of Tropism Results per CD4+ Category 100 80 Tropism Testing • Trofile test should be used whenever the use of a CCR5 antagonist is being considered (HIV VL must be >1000 copies/ml) • An enhanced sensitivity tropism assay has been developed to increase detection of minor CXCR4-using virus. Second generation Trofile assay has improved detection of minor variants 10-fold. First generation assay was a limitation in early maraviroc studies. • This is critical to the success/failure of these drugs. Typical HIV Tropism Patterns CCR5 tropic (R5) CXCR4 tropic (X4) Dual/mixed (D/M) Dual tropic Mixed tropism Trofile Assay • Developed by Monogram Biosciences and made available when FDA approved maraviroc, no other similar test available. • Test is used to determine whether a patient’s HIV uses the CCR5 or CXCR4 co-receptor (or both) to enter CD4 cells. • Similar to phenotype technology used for detecting HIV drug resistance. Assay amplifies HIV envelop gene from patient’s blood sample, HIV particles are made with the envelop protein and used to infect cells that contain CCR5 or CXCR4 co-receptor on the cell surface. Viral replication is measured in-vitro determining the tropism of the patient’s virus. Trofile™: Tropism Assay Clinical Trials • Tx-naïve (MERIT Trial) • Tx-experience (MOTIVATE I-II) Maraviroc Treatment-naïve • Rationale: 80-90% of tx-naïve patients harbor R5 virus • MERIT Trial: Non-inferiority study CBV bid + MVC 300mg bid vs CBV bid + EFV 600 qd • 48-week analysis found MVC did not meet noninferiority vs EFV (VL < 50 copies/ml) • MVC associated with higher CD4 increases • Limitation with sensitivity/specificity of initial Trofile assay Percentage of Patients with HIV-1 RNA <50 c/mL by Visit Includes all patients who were identified by the specified assay as R5 and received at least one dose of study medication; intent-to-treat (ITT) analysis 100 90 80 70 60 50 40 30 20 10 0 MERIT 100 MERIT-ES 90 Patients (%) 69% 64% 80 70 60 68% 68% 50 EFV + CBV (N=361) MVC + CBV (N=360) 40 30 EFV + CBV (N=303) MVC + CBV (N=311) 20 10 0 0 4 8 16 24 32 Time (weeks) 40 48 0 4 8 12 16 20 24 28 32 36 40 44 48 Time (weeks) Missing values classified as failures/non-responders Saag M, et al. ICAAC/IDSA 2008. Poster H-1232a MERIT Study – ESTA 48 weeks Enhanced Phenotype Tropism Assay: In Vitro Validation • Original assay validated in clinical trials – Did not detect minor CXCR4 species comprising <10% of population – Sensitivity in detecting CXCR4 HIV • 100% when 10% of population is CXCR4 • 85% when 5% of population is CXCR4 • Enhanced phenotype tropism assay validated using in vitro HIV env clones – Sensitivity in detecting CXCR4 HIV • 100% when 0.3% of population is CXCR4 • 81% when 0.1% of population is CXCR4 Trinh L, et al. 48th ICAAC. Washington, DC, 2008. Abstract H-1219. MERIT Week 48 Reanalysis by Enhanced Sensitivity Trofile Screening: Rationale and Methods • A more sensitive assay for the detection of CXCR4using virus might better select a population of patients who would respond to maraviroc, decreasing the number of failures associated with emergence of CXCR4-using HIV-1 • The enhanced sensitivity Trofile assay was performed on stored env expression vectors pools from all 721 patients in MERIT who had an R5 tropism result by the original Trofile assay • Testing was performed by Monogram Biosciences’ Clinical Reference Laboratory MERIT Study – ESTA 48 weeks Saag M, et al. ICAAC/IDSA 2008. Poster H-1232a 20 Summary: MVC in Treatment-naive Patients with R5 Virus • As the original Trofile assay is no longer available, the MERIT-ES reanalysis, while retrospective, was critical to inform appropriate clinical practice • The enhanced Trofile assay reclassified approximately 15% of patients as non-R5 HIV at screening • In this retrospective analysis, the lower bound of the one-sided 97.5% CI for the treatment difference, for both the <400 and <50 copies/mL endpoints, was above –10% (the prespecified noninferiority margin) • However, as this is a retrospective analysis, the confidence intervals presented here are for descriptive purposes only 21 MOTIVATE 1 & 2: Trial Design Randomization 1:2:2 MOTIVATE 1 N=601 MOTIVATE 2 N=474 Screening 6 weeks 0 OBT* + placebo 98/209 (46.9%) open-label MVC BID OBT* + maraviroc (150 mg† QD) 239/414 (57.7%) open-label MVC BID OBT* + maraviroc (150 mg† BID) 259/426 (60.8%) open-label MVC BID Week 48 Primary endpoint Week 96 Patients were stratified by enfuvirtide use and HIV-1 RNA < and ≥100,000 copies/mL Patient eligibility criteria: • R5 HIV-1 infection • HIV-1-RNA ≥5,000 copies/mL • Stable pre-study antiretroviral (ARV) regimen, or no ARVs for ≥4 weeks • Resistance to and/or ≥6 months’ experience with ≥one ARV from three classes (≥two for protease inhibitors [PIs]) Eligible patients (MVC non-failures, PBO failures or intolerance) were given the option to roll over to open-label MVC BID at end of blinded therapy (last patient week 48 visit). * OBT = optimized background therapy of 3–6 ARVs (PK boosting doses of ritonavir not counted as an ARV) † Patients receiving a PI (except tipranavir) and/or delavirdine in their OBT received 150 mg dose of MVC, all other patients received 300 mg dose of MVC MOTIVATE 1 and 2 Week 96 Safety and Efficacy Hardy WD et al. 9th Intl Cong. on Drug Ther in HIV Infection. Glasgow, UK, Nov 9-13 2008. Presentation O425 MOTIVATE 1 & 2: Percentage of Patients with HIV-1 RNA <50 copies/mL at Week 96 Includes all patients who received at least one dose of study medication MVC BID + OBT (N=426) MVC QD + OBT (N=414) 100 Placebo + OBT (N=209) 90 Option to switch to open-label MVC BID 80 Patients (%) 70 60 50 45.1% 40 43.7% 46.5% 41.3% 38.9% 43.5% 30 20 23.0% 16.7% 10 0 0 8 16 24 32 40 48 56 7.2% 64 72 80 88 96 Time (weeks) In this analysis, non-completers were categorized as failures MOTIVATE 1 and 2 Week 96 Efficacy Hardy WD et al. 9th Intl Cong. on Drug Ther in HIV Infection. Glasgow, UK, Nov 9-13 2008. Presentation O425 Mean Change from Baseline in CD4+ Cell Count PBO + OBT (N=209) in MOTIVATE 1 and 2 MVC QD + OBT (N=413) MVC BID + OBT (N=426) Mean change from baseline CD4+ cell count (cells/mm3) 140 124 116 120 100 80 60 61 40 20 0 n= PBO MVC QD n = MVC BID n = 0 4 8 12 16 20 24 28 Time (weeks) 32 186 362 386 205 399 412 206 405 417 206 407 418 206 407 418 206 407 418 206 407 418 MOTIVATE 1 and 2- Week 48 206 407 418 36 40 206 407 418 44 48 206 407 418 Virologic Response on Maraviroc-based Therapy is Greatest With a Background Regimen Containing Several Active Agents Patients (%) 100 90 80 70 60 50 40 30 20 10 0 MOTIVATE 1 and 2: Patients receiving maraviroc with an unchanged OBT across their period of randomized therapy (non-virologic failures excluded) Placebo + OBT MVC QD + OBT MVC BID + OBT 56 33 51 113 35 72 77 78 33 17 0 N *= wOBTSS • 51 70 41 76 <1 81 41 87 1-<2 ≥2 wOBTSS: The Weighted OBT Susceptibility Score -- a measure of OBT virologic activity Valdez et al. ICAAC/IDSA 2008, poster H-1221 More Severely Immunosuppressed Patients Achieve 200 CD4+ Cell with Maraviroc All treated patients with valid baseline and on-treatment measurements (LOCF) Patients with BL CD4+ cell count <200 Who achieved >200 at Wk 481 70 70 Placebo + OBT MVC QD + OBT MVC BID + OBT 60 47* 50 50 43 40 32* 30 Patients (%) 60 Patients (%) Patients with BL CD4+ cell count between 200 and 349 Who achieved >350 at Wk 48 40 20 10 10 0 0 235 250 59 116 117 38 30 20 N= 118 61 N= 64 *P=0.007 for MVC BID compared to placebo MOTIVATE 1& 2 – Week 48 Tressler R. 11th Annual Institute of Human Virology Meeting 2008 26 MOTIVATE Immunologic Subanalysis Summary • In MOTIVATE 1 and 2, highly TE patients receiving MVC (QD or BID) + OBT experienced early, rapid, greater, and persistent CD4+ cell count increases vs those receiving PBO + OBT (P=0.0182) • The CD4+ cell count advantage of MVC over PBO was driven not only by those patients who achieved virologic suppression, but also by those who never achieved this goal • Since treatment group was found to be associated with CD4+ cell rises, these findings together suggest that MVC increases CD4+ cell counts above and beyond what is expected for a given viral load reduction • Predictors of CD4+ cell count rises among MVC-treated patients were similar to those observed among non-MVC-treated patients Adverse Effects • Dose related: postural hypotension and dizziness • Long-term consequences of blocking chemokine receptor? - Hepatic abnormalities (aplaviroc) - Malignancies (vicriviroc study) • Most common reported SE higher than placebo were: diarrhea, fever, bronchitis, upper respiratory infection, back pain, dizziness, insomnia, cough, and rash. MOTIVATE: Safety Summary • No new or unique safety findings emerged – Similar safety profile as OBT alone • Adverse events • Severe adverse events • Laboratory abnormalities (including grade 3/4 transaminase elevations) • AIDS-defining events – Not associated with treatment-emergent X4 virus • Discontinuations due to adverse events (5%-6%) • Maraviroc + OBT is not associated with excess mortality compared with OBT alone Gulick RM, et al. N Engl J Med. 2008;359:1429-1441. Fatkenheuer G, et al. N Engl J Med. 2008;359:1442-1455. MOTIVATE 1 and 2 Pooled 48-week Safety Summary Placebo + OBT N = 209 MVC QD + OBT N = 414 Allcause n (%) Allcause n (%) Total exposure, patient-years Patients with AEs Txrelated n (%) 111 Txrelated n (%) MVC BID + OBT N = 426 Allcause n (%) 300 Txrelated n (%) 309 177 (84.7) 94 (45.0) 375 (90.6) 205 (49.5) 393 (92.3) 219 (51.4) 11 (5.3) 6 (2.9) 24 (5.8) 12 (2.9) 21 (4.9) 13 (3.1) Patients with grade 3 AEs 46 (22.0) 7 (3.3) 84 (20.3) 24 (5.8) 104 (24.4) 22 (5.2) Patients with grade 4 AEs 16 (7.7) 5 (2.4) 37 (8.9) 3 (0.7) 45 (10.6) 15 (3.5) 35 (16.7) 2 (1.0) 62 (15.0) 7 (1.7) 72 (16.9) 12 (2.8) Patients discontinuing due to AEs Patients with SAEs Patients with Category C events Deaths* 16 (7.7) 2 (1.0) 29 (7.0) 0 6 (1.4) 23 (5.4) 0 9 (2.1) 0 MOTIVATE 1 & 2: Incidence of LFT Abnormalities (Without Regard to Baseline) at Week 48 and End of Blinded Therapy Incidence (unadjusted) n (%) Incidence (adjusted) Event counts adjusted to 100 years of patient exposure Placebo + OBT MVC QD + OBT MVC BID + OBT Placebo + OBT MVC QD + OBT MVC BID + OBT AST: >3.0 x ULN 17 (8) 39 (10) 45 (11) 16.2 13.8 15.7 ALT: >3.0 x ULN 13 (6) 29 (7) 37 (9) 12.4 10.1 12.7 Total bilirubin: >1.5 x ULN 30 (14) 66 (16) 51 (12) 31.9 25.3 18.5 AST: >3.0 x ULN 19 (9) 45 (11) 46 (11) 13.3 9.4 9.5 ALT: >3.0 x ULN 15 (7) 37 (9) 39 (9) 10.0 7.8 7.8 Total bilirubin: >1.5 x ULN 31 (15) 68 (17) 54 (13) 23.8 16.0 11.3 Week 48 End of blinded therapy MOTIVATE 1 and 2 Week 96 Safety ULN, upper limit of normal Total patient-years of exposure to study drug at Week 48: Placebo + OBT 111; MVC QD + OBT 300; MVC BID + OBT 309 Total patient-years of exposure to study drug at at end of blinded therapy: Placebo + OBT 160; MVC QD + OBT 522; MVC BID + OBT 551 Summary: MVC in Treatment-experienced Patients with R5 Virus • Maraviroc (QD or BID) + OBT demonstrated significantly greater virologic suppression rates and increases from baseline in CD4+ cell counts at Weeks 24 and 48 compared to placebo + OBT in this combined analysis • Virologic suppression <50 copies/mL at Week 48 in the combined studies was preserved through Week 96 in 87% of patients receiving MVC BID + OBT • Maraviroc + OBT demonstrated a similar safety profile compared to placebo + OBT Maraviroc Pharmacokinetics • Can be taken with or without food • Primarily metabolized by CYP3A4 to inactive metabolites. • Approx. 20% renally eliminated • Half-life ≈ 15 hours (bid dosing) • Substrate of CYP3A4 and p-glycoprotein, but not an inducer or inhibitor, therefore MVC does not effect the plasma concentrations of other drugs. • MVC PK are affected by CYP3A4 and p-glycoprotein inhibitors and inducers • Pregnancy category B (no harm in animal models, but should only be used in pregnancy if clearly needed) MVC Dosing: Based on Two or Three Simple Questions Concomitant treatment Morning dose Evening dose 150 mg 150 mg Includes a potent CYP3A4/P-gp inhibitor For example: protease inhibitors +/- ritonavir (except tipranavir/r), elvitegravir/r*, delavirdine ketoconazole, itraconazole, clarithromycin, telithromycin, nefazadone YES Regardless of other agents in the regimen NO Includes a CYP3A4/P-gp inducer without a CYP3A4/P-gp inhibitor For example: efavirenz, etravirine, rifampicin, carbamazepine, oxcarbazepine, phenobarbital, phenytoin YES 300 mg 300 mg 300 mg 300 mg NO NO CYP3A4/P-gp inhibitors or inducers For example: NRTIs, enfuvirtide, nevirapine, tipranavir/r, raltegravir, SMX/TMP, ethinyl estradiol, levonergestrel, midazolam 300 mg 300 mg Note: Agents specifically studied with MVC are shown in italics * If elvitegravir/r is co-administered with a ritonavir-boosted PI, then the MVC dose should be adjusted based on MVC dosing recommendations for co-administration with that PI/r 34 Renal Dosing • Renal clearance accounts for 25% of total clearance Concomitant Medications Creatinine Clearance Dose Interval Potent CYP3A4 inhibitors 50-80 ml/min 30 - <50 ml/min <30 ml/min Every 24 hours Without potent CYP3A4 inhibitors 50 -80 ml/min 30 - <50 ml/min < 30 ml/min No dose adjustment required If co-administered with saquinavir/ritonavir 50 – 80 ml/min 30 - <50 ml/min < 30 ml/min Every 24 hours Every 48 hours Every 72 hours Advantages of CCR5 Antagonists • No cross resistance with other HIV medications, including enfuvirtide • Excellent option for treatment experienced pts • Co-receptor utilization (aka Viral Tropism) is associated with CD4 count (R5 more common when CD4>200) and antiretroviral treatment naïve patients. • Well tolerated • Immunologic benefit? Disadvantages of CCR5 Antagonists • Requires test to determine co-receptor tropism ($2,000) • Only active in ≈ 50% of tx-experienced pts • Blocks biologic receptor (long-term consequences?) Δ 32 mutants (1%) show no deleterious effects • Dosing based on co-administration of other medications (important for pharmacist)