* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Celiac Disease
Vaccination wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Crohn's disease wikipedia , lookup
Periodontal disease wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Gluten immunochemistry wikipedia , lookup
Ulcerative colitis wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Graves' disease wikipedia , lookup
Schistosomiasis wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Kawasaki disease wikipedia , lookup
Coeliac disease wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
Behçet's disease wikipedia , lookup
Ankylosing spondylitis wikipedia , lookup
Autoimmunity wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Neuromyelitis optica wikipedia , lookup
Inflammatory bowel disease wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Germ theory of disease wikipedia , lookup
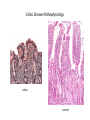
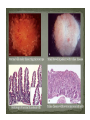
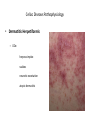
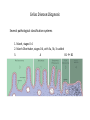
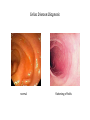
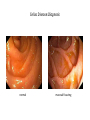
Celiac Disease Equipping primary care physicians to manage most patients Eric Poulin, MD First Light Health System Mora, Minnesota Celiac Disease:Objectives • Discuss definitions • Review pathophysiology and epidemiology • Discuss associated diseases • Equip primary care physicians to manage most patients Celiac Disease:Definitions • Classical CD • • • Atypical CD • • • • Typical GI symptoms Fully developed gluten-induced villous atrophy resulting in intestinal malabsorption Few or no GI symptoms Associated diagnoses, i.e. iron deficiency, osteoporosis, infertility Fully developed gluten-induced villous atrophy resulting in intestinal malabsorption Silent CD • • • • Asymptomatic No associated diagnoses Positive serologic testing in a higher risk patient Some degree of gluten-induced villous atrophy is present (or assumed to be present) Celiac Disease:Definitions classical atypical & silent Doc, what do I eat? Celiac Disease:Pathophysiology 1. Disorder of small bowel malabsorption. 2. Small bowel mucosa is damaged by inflammation, mediated by the immune system. 3. Immune system attack over time results in villous atrophy, crypt hyperplasia, and lymphocytic infiltration. Celiac Disease:Pathophysiology celiac normal Celiac Disease:Pathophysiology This inflammatory process can cause the “classic” symptoms: • • • • • • Abd pain Diarrhea Bloating Gas Fatigue Weight Loss (failure to thrive; delayed puberty) It can also cause a “classic” inflammatory skin rash, herpetiformis. dermatitis Celiac Disease:Pathophysiology • Dermatitis Herpetiformis – DDx: herpes simplex scabies neurotic excoriation atopic dermatitis Celiac Disease:Pathophysiology • Dermatitis Herpetiformis – Severely pruritic, erythematous, vesicular rash – Tends to occur in large symmetrical groups, on extensor surfaces: elbows, knees, scalp, buttocks, back of neck – Recurrent: lesions in various stages of healing – A “regular” skin biopsy is non-specific. – dermal microabscesses, with PMNs and eosinophils, at tips of dermal papillae – Send skin biopsy in special media to dermatopathologist for IgA immunofluoresence. Celiac Disease:Pathophysiology • Mucosal damage interferes with digestion and absorption Nutrient deficiency Resulting Disease/Symptom Iron Microcytic Anemia Calcium Osteoporosis, early onset Vit D Osteoporosis, and ? Vit B12, Folate Macrocytic Anemia, Peripheral Neuropathy Vit A Night Blindness “Nutrient Excess” Resulting Disease/Symptom Lactose Lactose Intolerance Fats Steatorrhea Celiac Disease:Pathophysiology Other deficiencies (B vitamins, micronutrients, magnesium, etc.) may be cause of non-specific neurological symptoms: – – – – – headaches ataxia poor memory depression seizures Celiac Disease:Pathophysiology • What triggers the immune system, the inflammatory reaction? • History – Willem Karel Dicke – Dutch pediatrician, 1944 wheat bread – Gluten identified about 10 years later by British Celiac Disease:Pathophysiology • When the gluten protein is ingested by a genetically susceptible person, an enzyme present in the small bowel mucosa, tissue transglutaminase, binds to and modifies the protein, making an antigen that is part “auto-” and part environmental (gluten). • This “modified” autoantigen is presented to both T cells (cell mediated) and B cells (humoral) which begin and continue the destructive inflammatory process. • Unlike other autoimmune diseases, however, celiac disease is reversible, by eliminating the environmental factor (gluten). Celiac Disease:Pathophysiology • The gluten protein is present in wheat, barley, and rye. • The gluten protein is present in “regular” oats due to cross-contamination in the field, in harvesting, and in processing. • How much gluten is needed to stimulate the immune response? – Very small quantities. • Studies show 10-50 parts per million (Fasano, 2007) • The USDA/FDA will add gluten to the “allergy declaration” at the bottom of ingredient lists using a 20 ppm cut-off. Celiac Disease:Pathophysiology • Remember: celiac disease is more similar to an autoimmune disease than an “intolerance” (i.e. lactose intolerance) or “sensitivity” or “allergy”. Elimination of gluten from the diet eventually ends the inflammatory response in most patients. – Many patients will become less “sensitive” and less symptomatic after several years of strict adherence to GFD. Celiac Disease:Epidemiology • Who is “genetically susceptible”? – Those with human leukocyte antigen (HLA) DQ2 or DQ8 receptors on their white blood cells—these genes are located on chromosome 6. – Other unidentified genetic programming is also involved. – Monozygotic twins show 70% concordance. – One of the highest risk factors is first-degree relatives. – Ethnic distribution: • highest risk – Europeans (Irish, Italians) • lowest risk – African, Japanese, Chinese Celiac Disease:Epidemiology • Prevalence in North America – Approximately 1:100, or 1% – Rises to 1:22 among first-degree relatives, almost 5% • Associated with other diseases (increased prevalence) – – – – – Type I Diabetes Down Syndrome & Turner Syndrome Autoimmune thyroiditis (Hashimoto’s, Graves, multi-nodular goiter) Microscopic Colitis Infertility Celiac Disease:Diagnosis • Newly Proposed Diagnostic Rule: Fasano’s 4 of 5: 1. 2. 3. 4. 5. Typical symptoms of celiac disease (clinical suspicion) High titers of TTG-IgA or endomysial-IgA (EMA) antibodies HLA-DQ2 or DQ8 genotypes Celiac enteropathy on small bowel biopsy Response to gluten-free diet Catassi, Fasano, American Journal of Medicine, 2010 Celiac Disease:Diagnosis • Serology: Antibody Sensitivity Specificity TTG-IgA 94% 98.7% EMA 93% 99.7% Lewis NR, Aliment Pharmacol Ther, 2006 Remember: – Patients must be eating gluten! – The higher the titer, the more convincing the result. – IgA testing is only accurate if IgA deficiency is ruled out. • Up to 5% of celiac patients are IgA deficient. – IgG class of antibodies is much less sensitive and specific. – IgA may not be reliable in infants and toddlers. Celiac Disease:Diagnosis • Genetic testing (HLA-DQ2 or DQ8): – High sensitivity: 99-100%. • Good for “ruling out” celiac disease. – Poor specificity: 57% • One third of the general US population is HLA-DQ2 + – Consider in: – infants and toddlers – Patients already eating a gluten-free diet Celiac Disease:Diagnosis • Small bowel biopsy • 4-6 (or more?) biopsies from 2nd or 3rd portion of duodenum, often random • Biopsy location can be guided by magnification, staining techniques, narrow band imaging (?) • Video capsule endoscopy: – sensitivity – 83%. specificity – 98%. no complications (no risk). Celiac Disease:Diagnosis Several pathological classification systems 1. Marsh, stages 0-4 2. March-Oberhuber, stages 0-4, with 3a, 3b, 3c added A B1 B2 3. Celiac Disease:Diagnosis normal flattening of folds Celiac Disease:Diagnosis normal mucosal fissuring Celiac Disease:Diagnosis • “Alternative” testing methods: – TTG-IgA levels in stool samples ? (EnteroLab) – TTG-IgA levels in saliva samples (Cyrex labs) • Italian study in 2010 with 4048 children, ages 6-8. Bonamico, M., J Pediatric Gastroenterol Nutr. 2011 Jan – 32 children saliva positive, 9 “borderline”. – 31/32 and 3/9 were positive with serum testing. – 28/34 had villous atrophy on biopsy. • 1 had “Marsh 1” pathology; 3 were not biopsied, but started on GFD – Prevalence in this study: • • 28 + 19 known CD in this population = 47/4048 = 1.2% Ratio of symptomatic to asymptomatic patients: 1:1.6 Celiac Disease:Diagnosis • Non-specific lab abnormalities – ALT, AST are mildly elevated – Albumin may be low • After diagnosis is made, remember to test for associated diseases: • DEXA for bone mineral density • TSH and thyroid exam for autoimmune thyroid disease • Hgb, and further w/u if anemic (iron studies, B12, RBC folate) Celiac Disease:Diagnosis • What is “response to gluten-free diet”? – Classic symptoms resolve • “Doc, I feel much better.” – Associated diagnoses improve • Iron tabs or meaty diet correct iron deficiency anemia. • Repeat BMD testing shows improvement on GFD and calcium+D. » younger patients can see dramatic BMD improvement – Diagnostic testing returns to normal, after 6 months on GFD • Previously high-titer serology is normal. • Repeat duodenal biopsies are normal. Celiac Disease:Treatment • But doc, what can I eat? Simple Low Carb Diet Simple GF Diet sweets breads pasta potatoes rice Wheat Barley Rye Oats • Infants – Breast feeding to 6 months – Delayed introduction of gluten to 6 months Celiac Disease:Treatment • Major difficulty of GF diet is eating out. – Cross-contamination • Deep fry oil • Preparation – No ingredient lists – Embarrassment among peers Celiac Disease:Treatment • Major difficulty of GF diet is home kitchen environment. – Cross-contamination • Separate items at meal-time • Separation of utensils and appliances (toaster, butter) • Non-food exposures – Children: Play-Doh – Adults: work environment (example: animal feed) Celiac Disease:Take home points—slide one • High index of suspicion in symptomatic GI disease, i.e. irritable bowel syndrome, chronic diarrhea, etc. • High index of suspicion in malabsorption diseases and autoimmune diseases. • Start out testing with TTG-IgA serology and consider the degree of abnormality in the result, and clinical risk factors. • Use Fasano’s 4 out of 5 diagnostic method: 1. 2. 3. 4. 5. Typical symptoms of celiac disease (clinical suspicion) High titers of TTG-IgA or endomysial-IgA (EMA) antibodies HLA-DQ2 or DQ8 genotypes Celiac enteropathy on small bowel biopsy Response to gluten-free diet Celiac Disease:Take home points—slide two • After diagnosis, remember: – – – – • Test for associated diseases Test immediate family members (higher risk group) Refer to a skilled dietician familiar with CD Repeat testing to confirm good control, after about 6 months Educate patients: celiac disease is a type of autoimmune disease: – The reason for strict gluten avoidance (over 20 ppm): immune system triggering inflammation damage. – Think of possible cross-contamination