* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Analysis - Wake Forest University
Serial digital interface wikipedia , lookup
Opto-isolator wikipedia , lookup
Oscilloscope history wikipedia , lookup
Valve RF amplifier wikipedia , lookup
Analog television wikipedia , lookup
Battle of the Beams wikipedia , lookup
Index of electronics articles wikipedia , lookup
Analog-to-digital converter wikipedia , lookup
Signal Corps Laboratories wikipedia , lookup
Dynamic range compression wikipedia , lookup
Signal Corps (United States Army) wikipedia , lookup
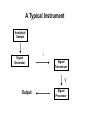
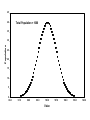
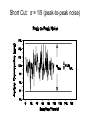
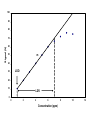
Chemical Analysis Qualitative Analysis Quantitative Analysis Determination “Analyze” a paint sample for lead “Determine” lead in a paint sample Bulk Material ↓ Sample ↓ Analytical Sample ↓ Analytical Matrix ↓ Analyte + Concomitants BLANK Same concomitants No analyte Difficult if not impossible to acquire a true blank INSTRUMENTAL ANALYSIS 1) Electroanalytical Chemistry 2) Spectrochemical Analysis 3) Chromatographic Separations A Typical Instrument Analytical Sample Signal Generator i Signal Transducer V Output Signal Processor Types of Signals 1. 2. 3. 4. 5. 6. Emission of Radiation Absorption of Radiation Scattering of Radiation Refraction of Radiation Diffraction of Radiation Rotation of Radiation 7. 8. 9. 10. 11. 12. 13. Electrical Potential Electrical Current Electrical Resistance Mass-to-charge Ratio Reaction Rate Thermal Properties Mass Signal Sources 1) 2) 3) 4) Analytical Signal Blank Signal Background Signal Dark Signal Measured Signal: A combination of these Analytical Figures of Merit “Indicate a characteristic of an instrumental technique for a given analyte” “7” Accuracy, Precision, Signal-to-Noise Ratio Sensitivity, Limit of Detection Linearity, Linear Dynamic Range Accuracy Indicates how close the measured value is to the true analytical concentration Requires a Standard Reference Material (SRM) of other official measure NIST: National Institute of Standards and Technology Accuracy Most commonly reported as percent error │Cm - Ct│ Ct x 100% where: Cm = measured concentration Ct = true concentration Precision Indicates the reproducibility of repetitive measurements of equivalent samples May be expressed as: 1. Standard Deviation (s or σ) 2. Relative Standard Deviation (RSD) 3. Confidence Limits Precision Standard Deviation For an infinite number of measurements (σ) For a finite number of measurements (s) Standard Deviation Note that both s and σ have the same units as the original values How many values should be obtained? Rule of thumb: 16 45 40 Total Population = 1000 35 Population 30 25 20 15 10 5 0 96.0 97.0 98.0 99.0 100.0 Value 101.0 102.0 103.0 104.0 1.00% How far is the measured mean from the true value? 0.90% 0.80% Error in Mean 0.70% 0.60% 0.50% 0.40% 0.30% 0.20% 0.10% 0.00% 0 5 10 15 20 25 30 Number of Samples 35 40 45 50 70.0% How far is s from σ? 60.0% Error in Std. Dev. 50.0% 40.0% 30.0% 20.0% 10.0% 0.0% 0 5 10 15 20 25 30 Number of Samples 35 40 45 50 Short Cut: σ ≈ 1/5 (peak-to-peak noise) Relative Standard Deviation RSD = σ/mean Where the mean may be the signal or the analyte concentration. RSD is a unit-less value, so σ must have the same units as the mean. RSD is often reported as %RSD, and may be used to compare different techniques. Confidence Limits Define an interval that encloses the true value (Ct) with a specified level of confidence. 1. Cm ± σ 66.7% Confidence Level 2. Cm ± 2σ 95% Confidence Level 3. Cm ± 3σ 99.0% Confidence Level Signal to noise Ratio (S/N) S/N = Sm/σ = 1/RSD Notes: 1. N = noise (σ) 2. S/N is unitless 3. Always try top maximize S/N 4. S/N is used to compare instruments 5. A plot of S/N versus an instrumental parameter reaches a maximum at the optimum value for that parameter Sensitivity Experimental slope of a calibration curve m = ΔS/ΔC Sensitivity is almost always specific for one particular instrument. 100 90 80 Signal (V) 70 60 50 m 40 LOD 30 20 10 LDR 0 0 2 4 6 Concentration (ppm) 8 10 12 Limit of Detection The analyte concentration yielding an analytical signal equal to 3 times the standard deviation in the blank signal. LOD = 3 x σbl / m By definition, the LOD has just one significant figure!! Linearity Measure of how well the observed data follows a straight line. SA = mC SA = Analytical Signal m = calibration sensitivity Remember SA = Stot - Sbl Linearity Plot log(S) versus log(C) log(SA) = log(m) + log(C) The slope of this plot should be 1.00 A calibration curve is defined as linear if the log-log plot has a slope in the range 0.95-1.05 Linear Dynamic Range The concentration range over which the calibration curve is linear Lower End → LOD Upper End Analyte Concentration where the observed signal falls 5% below the extrapolated line LDR Units are “orders of magnitude” or “decades” of analyte concentration LDR is easiest to observe on log-log plot If linearity is poor, define an analytically useful range (AUR) Other figures of merit may be calculated, but these 7 are sufficient. Selectivity and Resolution may be useful in cases where more than one analyte is determined in the same sample.