* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Sedimentary Materials
Survey
Document related concepts
Transcript
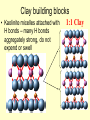
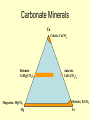
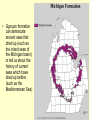
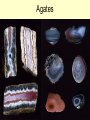
Sedimentary Minerals • We will focus on some minerals which form from precipitation of dissolved ions other minerals in sedimentary rocks are derived from the source rocks! • Clay, carbonate, and sulfate groups are key in sedimentary rocks – can ‘be’ the rock or cement fragments together! – SiO44-, CO32-, SO42- anionic groups, respectively • Also consider halides (anion is Cl- or F-) and mineralization of silica Clays Sheet Silicates – aka Phyllosilicates [Si2O5]2Sheets of tetrahedra micas talc clay minerals serpentine Phyllosilicates Sheet Silicates – aka Phyllosilicates [Si2O5]2Sheets of tetrahedra micas talc clay minerals serpentine Phyllosilicates •Clays talc pyrophyllite micas •Display increasing order and lower variability of chemistry as T of formation increases Clays • Term clay ALSO refers to a size (< 1mm = <10-6 m) • Sheet silicates, hydrous – some contain up to 20% H2O together with a layered structure and weak bonding between layers make them SLIPPERY WHEN WET • Very complex (even argued) chemistry reflective of specific solution compositions Major Clay Minerals • Kaolinite – Al2Si2O5(OH)4 • Illite – K1-1.5Al4(Si,Al)8O20(OH)4 • Smectites: – Montmorillonite – (Ca, Na)0.20.4(Al,Mg,Fe)2(Si,Al)4O10(OH)2*nH2O – Vermicullite - (Ca, Mg)0.30.4(Al,Mg,Fe)3(Si,Al)4O10(OH)2*nH2O – Swelling clays – can take up extra water in their interlayers and are the major components of bentonite (NOT a mineral, but a mix of different clay minerals) Clay building blocks • Kaolinite micelles attached with H bonds – many H bonds aggregately strong, do not expend or swell 1:1 Clay Clay building blocks • Slightly different way to deal with charge on the octahedral layer – put an opposite tetrahedral sheet on it… • Now, how can we put these building blocks together… 2:1 Clay Cement • Mixture of lime (CaO – made by roasting calcite) and silicates made by sintering limestone and clay • Ancient cement was just CaO – mixed with water to form portlandite (Ca(OH)2), which then slowly reacted with CO2 to reform calcite • Modern cement is mixed with water, form several Ca-Si phases that are more durable and don’t shrink as much Carbonate Minerals Ca Calcite, CaCO3 Dolomite CaMg(CO3)2 Magnesite, MgCO3 Mg Ankerite CaFe(CO3)2 Siderite, FeCO3 Fe Calcite Group • Variety of minerals varying by cation • Ca Calcite • Fe Siderite • Mn Rhodochrosite • Zn Smithsonite • Mg Magnesite Dolomite Group • Similar structure to calcite, but Ca ions are in alternating layers from Mg, Fe, Mn, Zn • Ca(Mg, Fe, Mn, Zn)(CO3)2 – Ca Dolomite – Fe Ankerite – Mn Kutnahorite Aragonite Group • Polymorph of calcite, but the structure can incorporate some other, larger, metals more easily (Pb, Ba, Sr) – Ca Aragonite – Pb cerrusite – Sr Strontianite – Ba Witherite • Aragonite LESS stable than calcite, but common in biological material (shells….) Calcite vs. Dolomite • dolomite less reactive with HCl calcite, has lower indices of refraction • dolomite more commonly euhedral and twinned • calcite commonly colorless • dolomite may be cloudy or stained by iron oxide • Mg spectroscopic techniques! • Different symmetry cleavage same, but easily distinguished by XRD Sulfate Minerals • More than 100 different minerals, separated into hydrous (with H2O) or anhydrous (without H2O) groups • Gypsum (CaSO4*2H2O) and anhydrite (CaSO4) are the most common of the sulfate minerals • Gypsum typically forms in evaporitic basins – a polymorph of anhydrite (g-CaSO4) forms when the gypsum is later dehydrated) Gypsum • Gypsum formation can demarcate ancient seas that dried up (such as the inland seas of the Michigan basin) or tell us about the history of current seas which have dried up before (such as the Mediterranean Sea) Halide Minerals • Minerals contianing halogen elements as dominant anion (Cl- or F- typically) • Halite (NaCl) and Sylvite (KCl) form in VERY concentrated evaporitic waters – they are extremely soluble in water, indicate more complete evaporation than does gypsum • Fluorite (CaF2) more typically occurs in veins associated with hydrothermal waters (F- in hydrothermal solutions is typically much higher – leached out of parent minerals such as biotites, pyroxenes, hornblendes or apatite) Sulfate Minerals II • Barite (BaSO4), Celestite (SrSO4), and Anglesite (PbSO4) are also important in mining. • These minerals are DENSE Barite =4.5, Anglesite = 6.3 (feldspars are ~2.5) Barite, Celestite, Anglesite • Metals bond with sulfate much more easily, and thus are generally more insoluble – they do not require formation in evaporitic basins • What do they indicate then? Ba, Pb, Sr – very low SO4 2- Lots of SO42Not very much Ba, Sr, Pb Just silica… • Chert – extremely fine grained quartz – Forms as nodules in limestone, recrystallization of siliceous fossils – Jasper – variety with hematite inclusions red – Flint – variety containing organic matter darker color • Chalcedony – microcrystaliine silica (very similar to low quartz, but different – it is yet uncertain how different…) typically shows banding, often colored to form an agate (rock formed of multiple bands of colored chalcedony) • Jasper – variety colored with inclusion of microcrystsalline oxides (often iron oxides = red) • Opal – a hydrogel (a solid solution of water in silica) – forms initially as water + silica colloids, then slowly the water diffuses into the silica making it amorphous (no XRD pattern!) – Some evidence opal slowly recrystallizes to chalcedony Opal - Gemstone Agates