* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Mineral
Survey
Document related concepts
Transcript
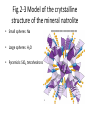
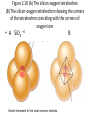
Chapter 2 Atoms, Elements, and Minerals Minerals • Mineralogy: study of minerals • Mineral: naturally occurring, crystalline (solid), inorganic substance that possesses a fairly definite chemical composition and a distinctive set of physical properties – Most are composed of 8 elements (silicon, oxygen compose 75% of the crust) – Over 4500 are known – 10 account for more than 90% of the crust Atoms & Elements Atoms: Smallest, electrically neutral assemblies of energy & matter Electrons: Negative charged particles that orbits the nucleus Nucleus: Core of the atom containing neutrons and protons Protons: Positive charged particles Neutrons: Neutrally charged particles Element: Defined by the number of protons (atomic number) Atomic Mass Number: Number of protons + neutrons Isotopes: Atoms containing different number of neutrons (same number of protons) Atomic Weight (Mass): Weight of an average atom Helium and Neon Atoms Fig. 2.6 Diagrammatic representation of (A) sodium and (B) chlorine ions Bonding Ionic Bonding: Electrons exchanged Covalent Bonding: Electrons shared, e.g. Carbon & Silicon Metallic Bonding: e.g. gold & iron, electrons free to move throughout the crystal Polymorphs: Different crystal structures with same chemical composition Fig.2-3 Model of the crytstalline structure of the mineral natrolite • Small spheres: Na • Large spheres: H2O • Pyramids: SiO4 tetrahedrons Figure 2.10 (A) The silicon-oxygen tetrahedron (B) The silicon-oxygen tetrahedron showing the corners of the tetrahedron coinciding with the corners of oxygen ions • A SiO4 –4 Atomic framework for the most common minerals B Figure 2.12 Common silicate structures. Arrows indicate directions in which structure repeats indefinitely • Si2O7 –6 Figure 2.13 Diagram of the crystal structure of olivine, as seen from one side of the crystal Fig. 2.14 Single-chain Silicate Structure • (A) Model of a singlechain silicate mineral • (B) The same chain silicate shown diagrammatically as linked tetrahedrons Fig. 2.22 (B) Mica • Sheet silicate Silicates • Quartz: SiO2, 3-D framework tetrahedra • Feldspar: most abundant mineral in crust – Plagioclase feldspar O, Si, Al, and Ca or Na – Potassium (orthoclase) feldspar O, Si, Al, and K • • • • • Single independent tetrahedron olivine Single chain pyroxene Double chain amphibole (e.g. hornblende) Sheet mica (e.g. muscovite & biotite) 3-D framework quartz Physical Properties of Minerals (Identification of Minerals) • Classified using physical & chemical properties – usually, only physical properties are used • 1. Crystal form: size & shape assumed by crystal faces when crystal has time & space to grow – External Crystal Form: set of faces that have a definite geometric relationship to one another. – Steno’s law: The angle between two adjacent faces in a mineral are always the same. • 2. Hardness: resistance to scratching – Mohs’ hardness scale: • Fingernail 2.5 • Penny 3 • Window glass or knife blade 5.5 • Steel file 6.5 Moh’s Hardness Scale • • • • • 1. 2. 3. 4. 5. talc gypsum calcite fluorite Apatite • • • • • 6. orthoclase feldspar 7. quartz 8. topaz 9. corundum 10. diamond Physical Properties • 3. Cleavage: tendency to break along definite planes • Fracture: way a mineral breaks – Splinter – Conchoidal fractures • 4. Color: reflecting light, not a reliable property because of impurities • 5. Streak: color of powder of mineral • 6. Luster: appearance of mineral’s surface in reflected light – Metallic – Nometallic • Vitreous (glassy) • Pearly • Greasy • Earthy/dull Physical Properties • 7. Specific gravity: ratio of mineral’s weight to an equal volume of water • 8. Taste: Not recommended • 9. Double (refraction) imaging: e.g. calcite • 10. Reaction to HCl: calcite & dolomite – CaCO3 + 2HCl CaCl2 + H2O + CO2 (g) • 11. Magnetism: • 12. Striations: Straight parallel line on flat surfaces, e.g. plagioclase feldspar Conditions of Mineral Formation • Geological – Precipitate from molten rock (magmas & lavas) – Precipitate in ocean water – Precipitate in springs, caves, lakes – Precipitate due to evaporation – Sublimation at volcanic vents from gases • Biological – Calcite in coral reefs – Magnetite in skulls – Sulfate minerals from bacteria