* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Survey
Document related concepts
Cell-penetrating peptide wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein adsorption wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Metalloprotein wikipedia , lookup
Transcript
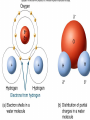
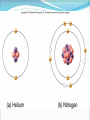
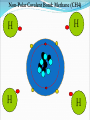
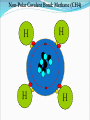
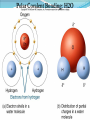
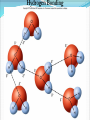
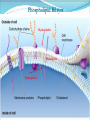
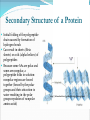
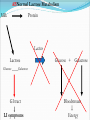
Chapter 2 Molecules of Life The Atom •Fundamental unit of matter •Nucleus •Protons: Positive charge; mass of 1 •Neutrons: No charge; mass of 1 •Electrons •Spin around the nucleus in orbitals (shells) •Negative charge; No mass http://www.yourdictionary.com/ahd/a/a0501900.html •Electrically negative: # of protons = # electrons Electrons •Electrons carry energy. How?? •Electrons are negatively charged as such, they are attracted to the positive charge in the nucleus. Meanwhile, electrons repel other electrons. REMEMBER… OPPOSITES ATTRACT and SAME REPELS •Electrons spin around the nucleus at various levels. They are attracted to the nucleus but repel each other, therefore it takes work to keep them in orbit. •Example is an apple in your hand. • Volumes of space that surround the •nucleus • Electrons move in orbitals Electron shells and electron orbitals Constants: The 1st shell in any atom can hold 2 electrons The 2nd shell in any atom can hold 8 electrons The 3rd shell in any atom can hold 8 electrons Shell P+N # of electrons each shell can hold First shell 2 Second shell 8 Third shell 8 Electron Movement •Electron shells = energy levels •Electron orbital = Volume of space around a nucleus where an electron is most likely to be found Useful Analogy: planets (electrons) ORBITING around the sun (nucleus) Why is it necessary to understand how electron orbitals work? This isn’t a Chemistry class, right?? Electrons and the energy they posses (their energy state) determine the chemical behavior of atoms thus, the losing, gaining or sharing of electrons is the BASIS FOR CHEMICAL REACTIONS IN WHICH CHEMICAL BONDS FORM (chemical bonds include hydrogen bonding, ionic bonding and covalent bonding). If electrons couldn’t lose or gain other electrons, or share with other electrons, chemical bonds would NOT form! Example, H2O Element a substance that cannot be reduced into a simpler component substance through a chemical process http://cougar.slvhs.slv.k12.ca.us/~pboomer/chemlectures/textass2/secondsemass.html How to Read the Periodic Table •Elements are arranged: LEFT to RIGHT and TOP to BOTTOM in order of increasing atomic mass. •Rows are arranged in periods Ex. H and He are in period 1 C and O are in period 2 The period number of an element = highest energy level an electron in that element occupies in an unexcited state Therefore, H and He have 1 electron shell C and O have 2 electron shells •Columns represent groups and families. •Each element symbol has 2 numbers listed: atomic number and atomic mass. Atomic Number 6 C Number of protons in the nucleus 12 Atomic Mass Number of protons and neutrons in the nucleus Fig. 3.3 Carbon Carbon Facts: •6 protons (Atomic # is 6) •6 neutrons (Atomic mass is 12…so, how do you get 6?) N = Atomic Mass - P •6 electrons (Atomic # is 6) •Is the first electron shell full (inactive)? Yes •Is the second electron shell full? No •How many unpaired orbitals does C have? 4 Carbon Carbon Facts: •How many chemical bonds can Carbon form with other atoms? 4 Can an element ever have a variable number of neutrons? Isotopes •Same atom but with a different # of neutrons, thus a different atomic mass Atomic number = # protons in the nucleus Atomic mass = # protons + # neutrons •Having a different number of neutrons in the nucleus DOES NOT change the chemical properties of an element BUT it DOES change the stability of the element!! Isotope Atomic # # protons 6 Atomic Mass #P+#N # protons # protons 6 6 #P + # N #P+#N 6 + 6 = 12 6 + 7 = 13 6 + 8 = 14 Medical Uses of Radioactive Isotopes •Short-lived isotopes are used clinically to diagnose pathological abnormalities/disease •Ex. Use of 99Tc for renal scan • 99 Tc (tracer) is introduced through your bloodstream •Kidney cells take up the radioactive tracer (isotope of Tc = 99Tc) •A camera detects emissions from the tracer and records them. •What makes 99Tc specific for kidney cells? The isotope is specific for a protein unique to kidney cells. Remember, electrons are the basis for chemical reactions!! So… if 99Tc has a different number of neutrons in its nucleus, the stability of the electrons in the other shell of that 99Tc atom are changed. There are 43 isotopes of Technitium! It just so happens that the particular stability of 99Tc seeks to form a chemical bond with this unique kidney protein. Matter •Any substance in the universe that has mass and occupies space •Matter is transformed through chemical bonding • Conservation of Matter = Matter cannot be created or destroyed but… it can be transformed •Use of an equation to show how matter is transformed: • Reactants •Sodium + Chloride Na+ + Cl- Products Sodium chloride NaCl Important Bonds in Biological Membranes •Way in which atoms link to one another to form molecules •Links are formed through the exchange of electrons •Atoms are driven to react to become more stable •Atomic stability is achieved by filling an outer electron shell •Non-reactive elements have full outer shells = INACTIVE •Types of chemical bonding •Ionic bonding •Covalent bonding •Hydrogen bonding Ionic Bonding •Creates ions (charged atoms): one atom loses electrons and becomes a (+) charged ion while another gains electrons and becomes (-) charged •Note: in charged atoms, the # of protons DOES NOT equal the # of electrons!!!! # Protons = # Electrons •Formed when atoms are attracted to each other by opposite electrical charges (i.e. magnet) •Two key properties of ionic bonding: •They are strong bond (although NOT the strongest) •They are non-directional Ionic Bonding Example: Table salt Reactants: Sodium atom has 1 lone electron in its outer orbital (Ax) + Chloride atom has 7 electrons in its outer orbital (Ax) Products: Sodium ION that has given up an electron from its outer shell + Chloride ION that has accepted an electron from Sodium and has included it in its outer shell Fig. 3.8 Both the sodium ion and the chloride ion are electrically attracted because of the opposite charges incurred by the altered electron orbitals. This electrical attraction results in the formation of an elaborate matrix resulting in a crystal of table salt. Covalent Bonds •Electrons are shared between atoms •Two key properties of covalent bonding: •VERY STONG!!! (strongest type of bond) •Directional •Carbon ALWAYS forms a covalent bond!!!!! •2 types: •Non-polar Covalent: electrons are equally shared •Generates hydrophobic molecules (“water hating”) •Polar Covalent: electrons are unequally shared •Generated hydrophilic bonds (”water loving”) Non-Polar Covalent Bond: Methane (CH4) H H H H Non-Polar Covalent Bond: Methane (CH4) H H H H Polar Covalent Bonding: H2O Hydrogen Bonding •Links a polar covalent molecule to another polar covalent molecule •Results in VERY WEAK bonding BUT because so many are formed, the complex as a whole is VERY STONG Hydrogen Bonding Solutions •A homogenous mixture of 2 or more substances •Solute = ingredient being dissolved •Solvent = substance that does the dissolving Example. You make a solution of water and salt. Which is the solute and which is the solvent? Solute = Salt Solvent = Water •Components of solutions Acids Bases Salts pH Components of Solutions, continued Acids •A substance that puts hydrogen ions (H+ )into a solution •Example: Hydrochloric Acid placed in water HCl + H2O Cl- + H+ Water HCl dissolved in water H H H H H H H H Components of Solutions, continued Bases •A substance that puts hydroxide ions (OH-) into solution •Example: Sodium Hydroxide dissolved in water NaOH + H2O Na+ + OH- Water NaOH dissolved in water OH OH OH OH OH OH OH OH Components of Solutions, continued Salts •A substance that puts other ions into solution (ions other than H+ and OH-) •Example: Sodium chloride dissolved in water NaCl + H2O Na+ + Cl- + H2O Cl Na Na Na Cl Cl Cl Na Cl Na Na Cl Na Cl Na Cl •Salts are formed when acids and bases are added to each other; this results in neutralization of the acid and base. HCl + NaOH NaCl + H2O (Acid) (Base) (Salt) (Water) Components of Solutions, continued pH •A logarithmic scale that measures the acidity of alkalinity (basicity) of a solution •Note: the difference between 2 units on the pH scale is 10, therefore, the difference between 3 pH units is… 100 •pH scale •Neutral : pH = 7 •Acidic : pH < 7 •Basic : pH > 7 •Buffers keep pH within normal limits Acidic Neutral Basic The Importance of Water to Life •Three quarters of the Earth’s surface is water •Two thirds of the human body is composed of water •All organisms require water •Since water is an essential part of life, it’s surprising that the bond that 2 atoms of H make with 1 atom of O is so weak. Actually, the bond that forms a single H20 molecule (which is what type of bond??) lasts only 1 / 100,000,000,000 of a second! •However, water molecules form extensive lattices with other water molecules. This occurrence leads to the important physical properties of water! Water 1. Water is a polar covalently bonded molecule that forms hydrogen bonds with other polar covalently bonded water molecules. 2. Universal solvent 3. Ice (solid water) is less dense than liquid ice. Ex. Ice floats in liquid water 4. Water has a high capacity to store heat. Water stabilizes Earth’s temperature (Remember, water comprises ¾ Earth’s surface. 5. Adhesion and cohesion Properties of Water •Bonds to hydrophilic substances and repels hydrophobic ones •Stabilizes temperature •Expands when it freezes •Cohesive •Dissolves substances Cohesion Since water is polar, it is attracted to other polar molecules. Cohesion occurs when the other polar molecule is water. Surface Tension Created by cohesion and due to the strong hydrogen bonding between the polar water molecules. Forming Macromolecules •Organic molecule •Formed by living organisms •Carbon-based core with functional groups attached •Functional group •Groups of atoms with special chemical properties •Confer specific chemical properties on the molecules that posses them •Ex. •Macromolecules •Potentially large molecules (Macro-) that are the building materials of cells. They are the material that makes up the body of cells and the machinery that runs within cells •Thousands of different types in an organism BUT the body is made of 4 types (protein, nucleic acid, carbohydrates, lipids) Five Principle Functional Groups Figure 3.17 More on Macromolecules Polymer: a molecule made of MANY chains of a similar subunit Monomer: a single molecule that is the BASIC building block of a macromolecule Monomers can combine to form a polymer View animation on Polymer formation http://science.nhmccd.edu/biol/dehydrat/dehydrat.html Dehydration Synthesis The process of FORMING a macromolecule Forms a COVALENT bond between two subunits: A hydroxyl (OH) group is removed from one subunit A hydrogen (H) is removed from the other subunit • Small molecule + small molecule • View animation large molecule + H20 Hydrolysis Reactions •The BREAKING up of a polymer •Adds a water molecule (H20) •H20 comes in and… •A hydrogen becomes attached to one subunit •A hydroxyl (OH) becomes attached to the other subunit •Results in the BREAKING of the covalent bond that previously held the macromolecule (polymer) together •Large molecule + H20 •View animation 2 small molecules Carbohydrates Contain C, H, O atoms (1:2:1 ratio) # Carbon atoms = # Oxygen atoms Hydrophilic Excellent for energy storage Why?? The C-H bonds store energy. When an organism requires an energy source, C-H bonds are the ones most often broken. This results in the release of stored energy. Comprise 1-2% of a cells mass 2 types: simple carbohydrates complex carbohydrates Simple Carbohydrates Monosaccharide Simple sugar Consists of one subunit; smallest carbs Ex. Glucose (C6H12O6) Also, fructose, ribose, deoxyribose See Figure 3.29 Disaccharide Result of linkage of two monosaccharides Ex. Sucrose, lactose, maltose See Figure 3.30 Complex Carbohydrates Polysaccharides Long chain polymers of sugars The body converts soluble sugars into insoluble forms (polysaccharides). These polysaccharides are then deposited throughout the body in specific storage areas. Preferred form of energy storage Plants: starch = glucose polysaccharide that plants use to store energy Animals: glycogen = highly insoluble macromolecule formed of glucose and polysaccharides that serves as stored energy •Utilized by plants and animals as structural polysaccharides (chitin and cellulose); linkage is unique such that the chains are not recognized by enzymes that normally break polysaccharide bonds. Lipids Contain C, H, and O Hydrophobic (held together by non-polar covalent bonds) Used as long term storage Contains MORE energy-rich C-H bonds than carbs Lipids I. Triglycerides (Fat) •Fats are synthesized from 2 components: 1. Fatty acid: long chain C and H atoms ending in a COOH group 2. Glycerol: a three C molecule; note, glycerol is an alcohol •Glycerol forms a backbone to which 3 fatty acids are attached via a dehydration reaction fat molecule •Provides long term energy storage, insulation Lipids, continued Triglycerides Saturated Fatty acids with ALL internal carbon atoms forming covalent bonds with two hydrogen atoms Animal source Solid at room temperature and body temp (37C) Unsaturated Fats with fatty acids that have double bonds between 1 or more pairs of carbon atoms Plant source Kink imparts a 30° bend: Liquid at room temperature Low melting point Why are unsaturated fats good while saturated fats are bad for your health? The C C bond in unsaturated fats creates a negative charge that causes the fat molecules to repel each other rather than stick together (as they do in long chain saturated fats). Hydrogenation Example: Margarine Margarine is formed from heating oil (unsaturated triglycerides) in the presence of a metal catalyst (aluminum) and hydrogen. That environment breaks the C C and replaces it with two hydrogen atoms producing very hard, saturated fats. Chemists vary the degree of time that hydrogenation occurs resulting in a product that is soft and spreadable (partially hydrogenated). N.B. Margarine is 10-50% trans fatty acids = BAD Margarine has been found to be contaminated with aluminum. Al is a causative agent in AD What is a trans-fatty acid? Trans fatty acids have hydrogen atoms on opposite sides of the double bonded carbons Cis fatty acids have hydrogen atoms that on the same side with each other The enzymes that metabolize fat can only metabolize cis fatty acids Butter is a saturated triglyceride. Why does butter soften as it melts, why doesn’t it instantly melt? Because the fatty acid chains that come off the glycerol backbone differ. Each different fatty acid has a different melting point. Common fats Saturated Palmitic acid Unsaturated Omega-3 Types of Lipids II. Phospholipid • Glycerol + 2 fatty acids + phosphate group • Polar group at one end (glycerol and phosphate) and highly nonpolar group at other end (fatty acid tails) • Ex. Cell membrane III. Steroid • 4-interlocking rings • Found in cell membranes • Ex. Cholesterol, hormones Basic structure of a triglyceride Basic structure of a phospholipid Phospholipid Bilayer Hydrophobic Hydrophilic Hydrophobic Protein Comprises 10-30% cell mass Functional roles (enzymes) and structural roles (collagen, keratin) All proteins are a long polymer chain of amino acid subunits small molecules, 20 total all 20 have a basic structure of a central carbon atom to which the 4 following are attached: hydrogen atom amino group (-NH2) carboxyl group (-COOH) an “R” group Amino Acids Nonpolar Hydrophobic Polar Uncharged Hydrophilic Polar Ionizable (Acidic) Hydrophilic Polar Ionizable (Basic) Hydrophilic How to make a protein Link specific amino acids together in a particular order Peptide bond = covalent bond that links 2 amino acids together Polypeptides = long chains of amino acids liked by peptide bonds Protein Structure Structure determines function What determines protein structure? Amino acid sequence of the protein Four levels of protein structure: Primary Secondary Tertiary Quaternary All levels of protein structure are ultimately determined by amino acid sequence!! Primary Structure of Protein The sequence of amino acids of a polypeptide chain Secondary Structure of a Protein Initial folding of the polypeptide chain caused by formation of hydrogen bonds Can result in sheets (Beta sheets) or coils (alpha helices) of polypeptides Because some AAs are polar and some are nonpolar, a polypeptide folds in solution: nonpolar regions are forced together (forced by the polar groups and their attraction to water resulting in the polar groups repulsion of nonpolar amino acids) http://kvhs.nbed.nb.ca/gallant/biology/biology.html Tertiary Structure of a Protein A folded and twisted molecule Repulsion by water forces nonpolar amino acids towards the interior leaving polar amino acids exposed to the exterior Quaternary Structure of a Protein Spatial arrangement of several component polypeptide chains http://www.chemsoc.org/exemplarchem/entries/2004/durham_mcdowall/images/1a3n-4-struct.png Denaturation What influences how a polypeptide folds in solution? The polar nature of the environment When the polar nature of the environment changes (↑ temp or ↓ pH), hydrogen bonding may be altered which may then cause unfolding of the protein, or denaturation. • Ex. Frying an egg Nucleic Acids Long polymers of nucleotides that serve as information storage devices of cells Nucleotides have 3 components: A five carbon sugar A phosphate group (PO4) An organic nitrogen-containing base • Polynucleotide chains - Chain of nucleic acids in which sugars are linked in a line by the phosphate groups …SUGAR – P – SUGAR – P - SUGAR – P … Nucleic Acids DNA and RNA DNA (deoxyribonucleic acid) Possible nucleotides: Adenine, Guanine, Cytosine, THYMINE Structure: 2 nucleotide strands = double helix RNA (ribonucleic acid) Possible nucleotides: Adenine, Guanine, Cytosine, URACIL Long, single strand How do nucleic acids function as information storage devices? Each nucleotide serves as a letter and each nucleic acid has different nucleotides (letters) Nucleotides Everyday Science •Lactose Intolerance – the inability to digest foods containing milk due to a lack of the lactase enzyme (enzyme, a protein that disrupts chemical bonds in other molecules allowing reactions to occur or preventing their occurrence). •Normally, milk sugar (lactose) is digested by the lactase enzyme. Lactase binds to lactose in milk and breaks the chemical bonds that are responsible for holding the sugar together. This allows the broken down sugars to pass through the bloodstream and be utilized by the body. •LI people lack the lactase enzyme, thus they cannot digest milk protein. This leads to a buildup of leading to nausea, cramps and bloaing. ABNormal Lactose Metabolism Milk Protein Lactase Lactose Glucose + Galactose Glucose _____Galactose GI tract LI symptoms Bloodstream Energy Questions 1. What is the strongest type of single bonded molecule? Covalent bond (both polar and non-polar types) 2. Isotopes have a different measure of stability when compared to their ‘parent’ element on the periodic table. True or False True 3. You can determine the number of neutrons present in an atom by subtracting the number of protons from the ____. Atomic mass 4. When preparing a solution, you accidentally add too much of an acidic component. This creates an excess of _____. The desired pH is 8; the pH you measure is 6. You decide that it shouldn’t make too much of a difference, you’re only 2 units off. What is wrong with this logic? H+, or Hydrogen ions A difference of 2 units on the pH scale correlates to a 100 fold more acidic solution. Therefore, your solution has 100 times more Hydrogen ions then the desired solution concentration. •Websites for additional info from today’s lecture: www.webelements.com Interactive periodic table http://web.buddyproject.org/web017/web017/pertab.html