* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CATEGORY A
Brucellosis wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Henipavirus wikipedia , lookup
West Nile fever wikipedia , lookup
Orthohantavirus wikipedia , lookup
Schistosomiasis wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Cross-species transmission wikipedia , lookup
Oesophagostomum wikipedia , lookup
Leptospirosis wikipedia , lookup
Sarcocystis wikipedia , lookup
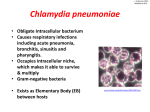
CHLAMYDIA, RICKETTSIA AND MYCOPLASMA Biological Agents/Diseases APPROVED CDC LIST (2003) (FEDERAL BIODEFENSE & BIOTERRORISM) CATEGORY A » Anthrax (Bacillus anthracis) » Botulism (Clostridium botulinum toxin) » Plague (Yersinia pestis) » Smallpox (variola major) » Tularemia (Francisella tularensis) » Viral hemorrhagic fevers (filoviruses [e.g., Ebola, Marburg] and arenaviruses [e.g., Lassa, Machupo]) CATEGORY B » Brucellosis (Brucella species) » Epsilon toxin of Clostridium perfringens » Food safety threats (e.g., Salmonella species, Escherichia coli O157:H7, Shigella) » Glanders (Burkholderia mallei) » Melioidosis (Burkholderia pseudomallei) » Psittacosis (Chlamydia psittaci) » Q fever (Coxiella burnetii) » Ricin toxin from Ricinus communis (castor beans) » Staphylococcal enterotoxin B » Typhus fever (Rickettsia prowazekii) » Viral encephalitis (alphaviruses [e.g., Venezuelan equine encephalitis, eastern equine encephalitis, western equine encephalitis]) » Water safety threats (e.g., Vibrio cholerae, Cryptosporidium parvum CATEGORY C » Emerging infectious diseases such as Nipah virus and hantavirus I. CHLAMYDIA IS THE PROTOTYPE OBLIGATE INTRACELLULAR PATHOGEN FOUR SPECIES: NATURAL HOSTS: 1. C. trachomatis: humans, mice and pigs 2. C. pneumoniae : humans and horses 3. C. psittaci: birds, mammals and humans 4. C. pecorum: cattle and sheep CHLAMYDIA LIFE CYCLE ELEMENTARY BODY OF CHLAMYDIA ELECTRON MICROSCOPY OF CHLAMYDIA LIFE CYCLE Properties of Chlamydial Particles Property Elementary body Reticulate body 0.2 – 0.4 μm 0.6 – 1.0 μm Rigid cell wall Yes No Extracellular stability Yes No Infective Yes No Induces phagocytosis Yes No Inhibits phagosome fusion with lysosome Yes No Toxic Yes No Metabolic activity No Yes Replication No Yes Size II. MEDICALLY IMPORTANT SPECIES III. CHLAMYDIA TRACHOMATIS There are at least 19 serovars (A-L) Different serovars are associated with distinct clinical signs Endemic trachoma is associated with serovars: A, B, Ba, and C Sexually transmitted disease (STD) is associated with serovars: D, Da, E, F, G, Ga, H, I, Ia, J, & K (D-K) Lymphogranuloma venereum (STD) is associated with serovars: L1, L2, L2a, and L3 (L1-L3) CHLAMYDIA TRACHOMATIS INCLUSION BODIES CHLAMYDIA TRACHOMATOUS Trachoma: Later stage of the formation of trachomatous pannus Women Increased susceptibility PID to HIV Cervical Infection (Usually assymptomatic) Fallopian tube Ectopic pregnancy Infertility Infection of infant Self-limiting Pneumonia eye infection (Conjunctivitis) Men Painful urination (discharge) Urethral infection (Non-gonorrheal urethritis) May be assymptomatic Reactive arthritis May cause Reiter’s syndrome (Usually in men) REITER’S SYNDROME Genital lesions, Conjunctivitis, papules on soles of feet, Arthritis CHLAMYDIA TRACHOMATIS SEROVARS L1, L2, & L3 LYMPHOGRANULOMA VENEREUM: Enlarged and ulcerative inguinal lymph node IV. CHLAMYDOPHILA PSITTACI Primarily a pathogen of birds, elementary bodies are excreted in droppings. Humans inhale elementary bodies, causing a flu-like illness (“parrot fever”- ornithosis) After an incubation period of 5 to 14 days the human develops headache, high fever, chills, malaise, myalgia, pulmonary consolidation, and nonproductive cough. Often dissemination to CNS. May cause encephalitis. Occasionally leads to death. Diagnosis is usually made by serology. Treatment with either Tetracyclines or erythromycin V. CHLAMYDOPHILA PNEUMONIAE Human to human transmission (No animal reservoir) Causes bronchitis, pneumonia and sinusitis. Infection in humans very common with several hundred thousand cases each year in the US. Causes a mild to severe “atypical pneumonia” similar to those caused by Mycoplasma pneumoniae and Legionella. Diagnosis by serology and by specific PCR. Tetracyclines and erythromycin are drugs of choice. A lot of published data suggest an etiologic association with atherosclerosis and coronary artery disease (Still in question). The organisms are ubiquitous. VI. RICKETTSIAE AND RICKETSIA-LIKE ORGANISMS ALL of these organisms are: 1. Small Gram negative coccobacilli 2. Obligate intracellular pathogens 3. Arthropod borne 4. With the exception of one group all have a non-human host and are zoonotic 5. They are all susceptible to Tetracyclines VII. RICKETTSIA & ORIENTIA Typhus and Spotted fevers These organisms are divided into distinct groups: 1. The spotted fever group with many species The most important species is Rickettsia rickettsii The etiology of ROCKY MOUNTAIN SPOTTED FEVER 2. The typhus Group: Rickettsia prowazekii causes epidemic typhus Rickettsia typhi causes murine typhus 3. The scrub typhus group: Orientia tsutsugamushi causes scrub typhus VIII. COXIELLA BURNETII: Q-FEVER IX. EHRLICHIA An Emerging Pathogen (Many Species) These are small Gram Negative rods (Rickettsia-like) They are obligate intracellular pathogens of either monocytes or PMNs but NOT erythrocytes (3 groups) All but one species are arthropod borne. E. sennetsu (restricted to Japan) causes disease in humans from eating raw fish Most species are transmitted from mammals to humans by ticks: Zoonoses). At least 3 forms of human disease (All are febrile illnesses): Sennetsu Human Monocytic Ehrlichiosis Human Granulocytic Ehrlichiosis Diagnosis can be difficult, best made using either serology or species specific DNA probes. X. MYCOPLASMA UREAPLASMA & L-Forms XI. MYCOPLASMA PNEUMONIAE