* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Bloodborne PathogenTraining
African trypanosomiasis wikipedia , lookup
Trichinosis wikipedia , lookup
Schistosomiasis wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Henipavirus wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Ebola virus disease wikipedia , lookup
Diagnosis of HIV/AIDS wikipedia , lookup
West Nile fever wikipedia , lookup
Epidemiology of HIV/AIDS wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Antiviral drug wikipedia , lookup
Marburg virus disease wikipedia , lookup
Microbicides for sexually transmitted diseases wikipedia , lookup
Leptospirosis wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
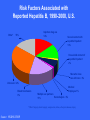
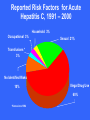
Elena Fracassa 993-5702 [email protected] www.oehs.wayne.edu Bloodborne diseases are only spread when blood (or certain other body fluids) from an infected person gets into the bloodstream of an uninfected person. This can happen when infected material enters through cuts or breaks in the skin, mucous membranes (eyes, nose), or directly into bloodstream, as with a needlestick. Universal Precautions Specific to blood & body fluids visibly contaminated with blood Applies to anyone regardless of infection status Does not apply to all body excretions/secretions Designed to reduce the risk of transmission of bloodborne pathogens (OSHA Standard) Bloodborne Diseases HIV: causes AIDS - no cure or vaccination HBV: Hepatitis B virus causes liver disease vaccination available HCV: Human Immunodeficiency Virus Hepatitis C virus causes liver disease no vaccination available Means of Transmission – Must Enter Body HBV, HIV virus present in blood, body fluids Sexual contact with an infected partner Sharing infected needles Accidentally cut/needlestick with a sharp object contaminated with infected blood, body fluids Infected blood or body fluid on skin especially with open cuts, sores Infected blood or body fluid splash to the eyes, nose, mouth. Bloodborne Diseases are NOT spread by: Kissing or hugging Sneezing Food or coughing or water Sharing Casual eating utensils, cups, etc. contact HIV Transmission Blood IV and body fluids serum semen vaginal secretions fluids around interenal organs/systems drug use vaginal or anal intercourse mother to child in utero HIV Symptoms night sweats loss of appetite weight loss fever skin rashes diarrhea fatigue swollen lymph nodes lack of resistance to infections HIV Transmission As of June 2004, there were 57 documented cases and 139 possible cases of occupationally acquired HIV among healthcare workers in the U.S. since reporting began in 1985. Centers for Disease Control Healthcare personnel with documented and possible occupationally acquired AIDS/HIV infection, by occupation, as of December 2002. No new documented cases of occupationally acquired HIV/AIDS have been reported since December 2001. One new case of possible occupational transmission has been reported. Occupation Documented Possible Nurse 24 35 Laboratory worker, clinical 16 17 Physician, nonsurgical 6 12 Laboratory technician, nonclinical 3 - Housekeeper/maintenance worker 2 13 Technician, surgical 2 2 Embalmer/morgue technician 1 2 Health aide/attendant 1 15 Respiratory therapist 1 2 Technician, dialysis 1 3 Dental worker, including dentist - 6 Emergency medical technician/paramedic - 12 Physician, surgical - 6 Other technician/therapist - 9 Other healthcare occupation - 5 57 139 Total HIV Exposure Risk Rate of seroconversion after needlestick exposure to infective material from HIV+ person is 0.3% or about 1 in 300. HIV in high concentration during period prior to antibody development. Much less infective than HBV, HCV, Herpes HIV Transmission in Healthcare Workers Factors Deep associated with HIV transmission: injury Device visibly contaminated with source patient’s blood Procedures involving a needle placed directly in a vein or artery Terminal illness in source patient No zidovudine prophylaxis Hepatitis A Facts Fecal-oral transmission – NOT bloodborne Spread through contaminated water, seafood, or infected food handlers. Incubation Children period from 15-45 days. usually have no symptoms. Adults may have fatigue, nausea, fever, jaundice. Hepatitis A Infection clears up over a few weeks to months, no chronic problems result. Vaccination recommended if outbreak occurs or for travel to some foreign countries. Routine vaccination in all children recommended as of May 2006 by the CDC Advisory Committee on Immunization Practices. Reported Cases of Hepatitis A in U.S. 45 1995: Vaccine Licensed 40 1996: ACIP recommendations Rate per 100,000 35 30 1999 ACIP recommendations 25 20 15 10 5 0 52 56 60 64 68 72 76 Year Source: NNDSS, CDC 80 84 88 92 96 2002 Concentration of Hepatitis A Virus in Various Body Fluids Body Fluids Feces Serum Saliva Urine 100 102 104 106 Infectious Doses per mL Source: Viral Hepatitis and Liver Disease 1984;9-22 J Infect Dis 1989;160:887-890 108 1010 Hepatitis B Virus of the liver – most common bloodborne disease Inflammation Symptoms range from flu-like to none at all symptoms – person can still be infectious and can spread the disease No Risk Factors Associated with Reported Hepatitis B, 1990-2000, U.S. Other* Injection drug use 14% 15% Sexual contact with hepatitis B patient 13% Household contact of hepatitis B patient 2% Men who have sex with men 6% Unknown 32% Blood transfusion 0% Medical Employee 1% Multiple sex partners Hemodialysis 0% 17% *Other: Surgery, dental surgery, acupuncture, tattoo, other percutaneous injury Source: NNDSS/VHSP Hepatitis B Facts Incubation period from 28-160 days Symptoms may include: loss of appetite fatigue fever possible jaundice and dark urine HBV is much greater risk on the job than HIV Hepatitis B Facts How HBV is transmitted: cut with sharp, contaminated object, needlestick splashes contact human to eyes/nose/mouth with broken skin bites Hepatitis B Facts Fluids that pose risk of infection: blood body and blood products fluids containing visible blood semen and vaginal secretions breast milk saliva (through a human bite) Concentration of Hepatitis B Virus in Various Body Fluids HIGH: blood, serum, wound exudates MODERATE: LOW semen, vaginal fluid, saliva / NOT DETECTABLE: urine, feces, sweat, tears, breast milk Hepatitis C Virus Identified in 1988, formerly called non-A non-B hepatitis - called “silent epidemic” Blood supply not tested until early 90s. Incubation period from 2-26 weeks. Most people never have symptoms Hepatitis C Virus Spread primarily through blood/blood products. Most likely not spread sexually. 80-85% There of cases become chronic is no vaccine for HCV. Based on limited studies, risk for infection after needlestick is approximately 1.8%. Risk Factors Associated with Transmission of HCV • Illegal injection drug use • Transfusion or transplant from infected donor • Occupational exposure to blood – Mostly needle sticks • Iatrogenic (unsafe injections) • Birth to HCV-infected mother • Sexual/household exposure to anti-HCV positive contact • Multiple sex partners Reported Risk Factors for Acute Hepatitis C, 1991 – 2000 Household 3% Occupational 3% Sexual 21% Transfusions * 3% No Identified Risks 10% Illegal Drug Use 60% *None since 1994 HBV Vaccination Administered in 3 shots over 6 months. Recombinant vaccine is yeast derived - no chance of infection from vaccination. Provides long term protection against HBV for 96% of healthy adults: no booster recommendation from CDC at this time. Post exposure vaccination is 70-88% effective when started within one week. Hepatitis B in Healthcare Workers (HCWs) in the U.S. Prior to vaccination, it was estimated that more than 12,000 HCWs were occupationally infected with HBV annually, resulting in 250 deaths. 1983 – Incidence of HBV among HCWs was 3 times higher than in the general population. By 1995 it was 5 times lower. Advent of HBV vaccine was a major advance in preserving health and lives of HCWs. Source: Arch Intern Med 1997; 157:2601-2605 What is an “Occupational Exposure” Contact with blood or other potentially infectious materials through: needlestick or cut with sharp, contaminated object contact with broken skin through cuts, rashes, other breaks in skin splashes to eyes, nose, mouth If there are no infiltrations of mucous membranes or open skin surfaces, it is not considered an occupational exposure. Responding to Exposures Thoroughly wash affected area. Do not wash with bleach or other strong cleaners Report incident to supervisor and get immediate medical attention. Complete appropriate report of injury forms. Postexposure follow-up Medical Evaluation Confidential Route of exposure (i.e. needlestick, splash to eyes, etc.) Source individual identification and testing Exposed individual blood testing Postexposure prophylaxis as recommended by U.S. public health service (CDC) Counseling Evaluation of illnesses Postexposure Follow-up Physician should follow the updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV and HIV and Recommendations for Postexposure Prophylaxis Postexposure Follow-up Health care professionals who evaluate employees after an exposure shall be given the following information: Description of employees duties as related to the exposure incident Documentation of the route and circumstances surrounding the exposure Results of the source individual’s blood testing Relevant medical records (including vaccine) Description of PPE used or to be used Postexposure Follow-up Employer shall obtain and provide to exposed employee a copy of the health care professional’s written opinion limited to: Any limitation on employee use of PPE HBV vaccine information Statement that employee has been informed of test results and medical conditions that may have resulted from exposure Personal Protective Equipment (PPE) Protective Lab coat clothing Disposable latex or non-latex exam gloves: change when torn or contaminated. Change between patients Wash hands inbetween Personal Protective Equipment Face protection: safety glasses/goggles worn with face mask if risk of aerosols. Other appropriate PPE if necessary: gown, face shield, booties,etc. PPE should not leave the work area! Hand Washing Before & after contact Before & after procedures After removing gloves After using restroom Anytime your hands are soiled Personal protective equipment shouldn’t leave the work area! Good Handwashing Warm, running water w/mild, preferably liquid soap, not required to be antibacterial Rub hands together vigorously for at least 15 seconds: scrub between fingers, under nails, tops & palms of hands Rinse with warm, running water Dry with disposable paper towel Use lotion to prevent chapping of hands Bacterial Reduction Ability of Hand Hygiene Agents to Reduce Bacteria on Hands % 99.9 Time After Disinfection log 0 60 180 minutes 3.0 99.0 2.0 90.0 1.0 0.0 0.0 Alcohol-based handrub (70% Isopropanol) Antimicrobial soap (4% Chlorhexidine) Baseline Plain soap Adapted from: Hosp Epidemiol Infect Control, 2nd Edition, 1999. Alcohol-based handrub is better than handwashing at killing bacteria. Across the top of this graph is the amount of time after disinfection with the hand hygiene agent. Left axis shows the percent reduction in bacterial counts. Hand Moisturizers and Lotions ONLY USE facility-approved/supplied lotions Some lotions may make medicated soaps less effective Some lotions cause breakdown of latex gloves Lotions can become contaminated with bacteria if dispensers are refilled ~ Do not refill lotion bottles ~ Survival on Surfaces HIV is weak and dies rapidly upon exposure to air. It doesn’t live in dried material. Hepatitis Live viruses are hardy. hepatitis B virus has been found in dried up to 14 days old. Cleaning and Decontamination Wear gloves and other appropriate PPE. Clean spills with disposable towels and a hot soap and water solution. Disinfect with a FRESH 1:10 bleach solution: inactivates HIV and hepatitis virus Tuberculocidal cleaners may also be used. Alcohol doesn’t destroy HBV or HCV. Dispose of contaminated items properly, remove PPE and WASH HANDS! Needlestick Safety and Prevention Act Only needle-locking syringes or disposable syringe-needle units (i.e., needle is integral to the syringe) are used for injection or aspiration of infectious materials. Syringes which re-sheathe the needle, needleless systems, and other safety devices are used when appropriate. Examples of Safe Needle Devices “Self-sheathing” needle device. “Retractable” needle device. Add on device – flip over to “cap” needle after use. “Add-on” SHARPS safety features Attached to syringe needle Attached to blood tube holder SHARPS Precautions Used disposable needles must not be bent, sheared, broken, recapped, removed from disposable syringes, or otherwise manipulated by hand before disposal. Always dispose of needles in SHARPS containers! SHARPS waste SHARPS containers must be used for all SHARPS – regardless of contamination Don’t overfill containers! Locate containers conveniently. Never recap, bend, or break needles! Dispose of entire unit together. Non-Sharp Biological Waste Waste containers must be: leak-proof labeled with biohazard word and symbol closable Follow the rules of your workplace! Practice Universal Precautions! Treat all blood, body fluids, tissue, etc. as if it is known to be infectious. Use good hygiene - wash hands frequently! Wear personal protective equipment. Dispose of SHARPS properly! Questions? Wayne State University Office of Environmental Health & Safety 5425 Woodward, Suite 300 Detroit, MI 48202 313.577.1200 www.oehs.wayne.edu