* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Antiarrythmic drugs
Cardiac contractility modulation wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Myocardial infarction wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Jatene procedure wikipedia , lookup
Electrocardiography wikipedia , lookup
Atrial fibrillation wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
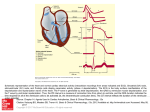
Antiarrhythmic Drugs Arrhythmia Heart condition where disturbances in Pacemaker impulse formation Contraction impulse conduction Combination of the two Results in rate and/or timing of contraction of heart muscle that is insufficient to maintain normal cardiac output (CO) To understand how antiarrhythmic drugs work, need to understand electrophysiology of normal contraction of heart Normal heartbeat and atrial arrhythmia Normal rhythm Atrial arrhythmia AV septum Ventricular Arrhythmia Ventricular arrhythmias are common in most people and are usually not a problem but… VA’s are most common cause of sudden death Majority of sudden death occurs in people with neither a previously known heart disease nor history of VA’s Medications which decrease incidence of VA’s do not decrease (and may increase) the risk of sudden death treatment may be worse then the disease! Electrophysiology - resting potential A transmembrane electrical gradient (potential) is maintained, with the interior of the cell negative with respect to outside the cell Caused by unequal distribution of ions inside vs. outside cell Na+ higher outside than inside cell Ca+ much higher “ “ “ “ K+ higher inside cell than outside Maintenance by ion selective channels, active pumps and exchangers Cardiac Action Potential Divided into five phases (0,1,2,3,4) Phase 4 - resting phase (resting membrane potential) Phase cardiac cells remain in until stimulated Associated with diastole portion of heart cycle Addition of current into cardiac muscle (stimulation) causes Phase 0 – opening of fast Na channels and rapid depolarization Drives Na+ into cell (inward current), changing membrane potential Transient outward current due to movement of Cl- and K+ Phase 1 – initial rapid repolarization Closure of the fast Na+ channels and outflow of K Phase 0 and 1 together correspond to the R and S waves of the ECG Cardiac Action Potential Phase 2 - plateau phase sustained by the balance between the inward movement of Ca+ and outward movement of K + Has a long duration compared to other nerve and muscle tissue Normally blocks any premature stimulator signals (other muscle tissue can accept additional stimulation and increase contractility in a summation effect) Corresponds to ST segment of the ECG. Phase 3 – repolarization K+ channels remain open, Allows K+ to build up outside the cell, causing the cell to repolarize K + channels finally close when membrane potential reaches certain level Corresponds to T wave on the ECG ECG (EKG) showing wave segments Contraction of atria Contraction of ventricles Repolarization of ventricles In normal atrial, Purkinje, and ventricular cells, the action potential upstroke (phase 0) is dependent on sodium current. From a functional point of view, it is convenient to describe the behavior of the sodium current in terms of three channel states). The cardiac sodium channel protein has been cloned, and it is now recognized that these channel states actually represent different protein conformations. In addition, regions of the protein that confer specific behaviors, such as voltage sensing, pore formation, and inactivation, are now being identified. The gates described below and in Figure represent such regions. Depolarization to the threshold voltage results in opening of the activation (m) gates of sodium channels If the inactivation (h) gates of these channels have not already closed, the channels are now open or activated, and sodium permeability is markedly increased, greatly exceeding the permeability for any other ion. Extracellular sodium therefore diffuses down its electrochemical gradient into the cell, and the membrane potential very rapidly approaches the sodium equilibrium potential, ENa (about +70 mV when Nae = 140 mmol/L and Nai = 10 mmol/L). This intense sodium current is very brief because opening of the m gates upon depolarization is promptly followed by closure of the h gates and inactivation of the sodium channels Most calcium channels become activated and inactivated in what appears to be the same way as sodium channels, but in the case of the most common type of cardiac calcium channel (the "L" type), the transitions occur more slowly and at more positive potentials. The action potential plateau (phases 1 and 2) reflects the turning off of most of the sodium current, the waxing and waning of calcium current, and the slow development of a repolarizing potassium current. Final repolarization (phase 3) of the action potential results from completion of sodium and calcium channel inactivation and the growth of potassium permeability, so that the membrane potential once again approaches the potassium equilibrium potential. The major potassium currents involved in phase 3 repolarization include a rapidly activating potassium current (IKr) and a slowly activating potassium current (IKs). These two potassium currents are sometimes discussed together as "IK.". It is noteworthy that a different potassium current, distinct from IKr and IKs, may control repolarization in sinoatrial nodal cells. This explains why some drugs that block either IKr or IKs may prolong repolarization in Purkinje and ventricular cells, but have little effect on sinoatrial nodal repolarization Mechanisms of Arrhythmias Many factors can precipitate or exacerbate arrhythmias: ischemia, hypoxia, acidosis or alkalosis, electrolyte abnormalities, excessive catecholamine exposure, autonomic influences, drug toxicity (eg, digitalis or antiarrhythmic drugs), overstretching of cardiac fibers, and the presence of scarred or otherwise diseased tissue. However, all arrhythmias result from (1) disturbances in impulse formation, (2) disturbances in impulse conduction, or (3) both. Disturbances of Impulse Formation The interval between depolarizations of a pacemaker cell is the sum of the duration of the action potential and the duration of the diastolic interval. Shortening of either duration results in an increase in pacemaker rate. The more important of the two, diastolic interval, is determined primarily by the slope of phase 4 depolarization (pacemaker potential). Vagal discharge and receptor-blocking drugs slow normal pacemaker rate by reducing the phase 4 slope (acetylcholine also makes the maximum diastolic potential more negative). Acceleration of pacemaker discharge is often brought about by increased phase 4 depolarization slope, which can be caused by hypokalemia,. adrenoceptor stimulation, positive chronotropic drugs, fiber stretch, acidosis, and partial depolarization by currents of injury . Latent pacemakers (cells that show slow phase 4 depolarization even under normal conditions, eg, some Purkinje fibers) are particularly prone to acceleration by the above mechanisms. However, all cardiac cells, including normally quiescent atrial and ventricular cells, may show repetitive pacemaker activity when depolarized under appropriate conditions, especially if hypokalemia is also present. Afterdepolarizations (the Figure below) are depolarizations that interrupt phase 3 (early afterdepolarizations, EADs) or phase 4 (delayed afterdepolarizations, DADs). EADs are usually exacerbated at slow heart rates and are thought to contribute to the development of long QT-related arrhythmias (see Molecular & Genetic Basis of Cardiac Arrhythmias). DADs on the other hand, often occur when intracellular calcium is increased. They are exacerbated by fast heart rates and are thought to be responsible for some arrhythmias related to digitalis excess, to catecholamine, and to myocardial ischemia Disturbances of Impulse Conduction Severely depressed conduction may result in simple block, eg, atrioventricular nodal block or bundle branch block. Because parasympathetic control of atrioventricular conduction is significant, partial atrioventricular block is sometimes relieved by atropine. Another common abnormality of conduction is reentry (also known as "circus movement"), in which one impulse reenters and excites areas of the heart more than once In order for reentry to occur, three conditions must coexist, as indicated in Figure beiowe: (1) There must be an obstacle (anatomic or physiologic) to homogeneous conduction, thus establishing a circuit around which the reentrant wavefront can propagate; (2) there must be unidirectional block at some point in the circuit, ie conduction must die out in one direction but continue in the opposite direction (as shown in the Figure, the impulse can gradually decrease as it invades progressively more depolarized tissue until it finally blocks—a process known as decremental conduction); and (3) conduction time around the circuit must be long enough so that the retrograde impulse does not enter refractory tissue as it travels around the obstacle, ie the conduction time must exceed the effective refractory period. Importantly, reentry depends on conduction that has been depressed by some critical amount, usually as a result of injury or ischemia. If conduction velocity is too slow, bidirectional block rather than unidirectional block occurs if the reentering impulse is too weak, conduction may fail, or the impulse may arrive so late that it collides with the next regular impulse. On the other hand, if conduction is too rapid, ie almost normal, bidirectional conduction rather than unidirectional block will occur. Even in the presence of unidirectional block, if the impulse travels around the obstacle too rapidly, it will reach tissue that is still refractory = 10 mmol/L). This intense sodium current is very brief because opening of the m gates upon depolarization is promptly followed by closure of the h gates and inactivation of the sodium channels Slowing of conduction may be due to depression of sodium current, depression of calcium current (the latter especially in the atrioventricular node), or both. Drugs that abolish reentry usually work by further slowing depressed conduction (by blocking the sodium or calcium current) and causing bidirectional block. In theory, accelerating conduction (by increasing sodium or calcium current) would also be effective, but only under unusual circumstances does this mechanism explain the action of any available drug. Lengthening (or shortening) of the refractory period may also make reentry less likely. The longer the refractory period in tissue near the site of block, the greater the chance that the tissue will still be refractory when reentry is attempted. (Alternatively, the shorter the refractory period in the depressed region, the less likely it is that unidirectional block will occur.) Thus, increased dispersion of refractoriness is one contributor to reentry, and drugs may suppress arrhythmias by reducing such dispersion Antiarrhythmic drugs Biggest problem – antiarrhythmics can cause arrhythmia! Example: Treatment of a non-life threatening tachycardia may cause fatal ventricular arrhythmia Must be vigilant in determining dosing, blood levels, and in follow-up when prescribing antiarrhythmics Classification of antiarrhythmics (based on mechanisms of action) Class I – blocker’s of fast Na+ channels Subclass IA [markedly Na block] Cause moderate Phase 0 depression Prolong repolarization Increased duration of action potential Includes Quinidine – 1st antiarrhythmic used, treat both atrial and ventricular arrhythmias, increases refractory period Procainamide - increases refractory period but side effects Disopyramide – extended duration of action, used mainly for treating ventricular arrhythmias Classification of antiarrhythmics (based on mechanisms of action) Subclass IB [inhibitory effect] Weak Phase 0 depression Shortened repolarization Decreased action potential duration Includes Lidocane (also acts as local anesthetic) – blocks Na+ channels mostly in ventricular cells, also good for digitalis-associated arrhythmias Mexiletine - oral lidocaine derivative, similar activity Phenytoin – anticonvulsant that also works as antiarrhythmic similar to lidocane Tocainide Classification of antiarrhythmics (based on mechanisms of action) Subclass IC [very major block] Strong Phase 0 depression No effect of repolarization No effect on action potential duration Includes Flecainide (initially developed as a local anesthetic) Slows conduction in all parts of heart, Also inhibits abnormal automaticity Propafenone Also slows conduction Weak β – blocker Also some Ca2+ channel blockade CLASS IA Quinidine ;the first antiarrhythmic Slow conduction and increase refractoriness in the retrograde fast pathway limb of AV nodal tachycardias and over the accessory pathway Slow ventricular response in WPW syndrom Inhibit peripheral and myocardial α-adrenergic receptor so cause hypotension with IV administration Inhibit muscarinic receptor increase sympathetic tone that may explain part of proarrhythmic effect Indication The use of quinidine for atrial flutter and fibrillation has been replaced by other Drugs It can be used for ventricular tachyarrhythmia but the proarrhythmic effect ;non cardiac side effect and drug interaction have led to dramatic reduction in its use Caution Idoisyncrasy and is best prevented by a test dose of 0.2 g also by serial measurements of QRS duration Side effect Diarrhea;nausea;headache;dizziness with a high rate of discontinuation and Hypersensitivity reaction CI QT prolongation with VT or prior therapy with drugs predispose torsade de pointes Procainamide Like quinidine but does not prolong the QT interval to the same extent ; Has less interaction with muscarinic receptors ;direct sympathetic inhibition (vasodilation) Indication Supraventricular including WPW syndrom and ventricular arrhythmias including VT In sustained VT procainamide is more effective than lidocaine at the cost of QRS and QT widening Giving orally ;not for long time due to the short half-life and the long-term danger of Lupus syndrom Giving IV if lidocaine failed Advantage over quinidine less side effect for GI;QRS prolongation or torsades;hypotension No interaction with digoxin Side effect Early ;rash and fever later;arthralagia; rash and lupus syndrom With IV giving there is more hypotension and QRS and QT widening CI severe renal or cardiac failure Disopyramide Like quinidine also it prolong QRS and QT intervals (risk of torsade) It improves AV nodal conduction due to its anticholinergic effect Unlike quinidine its use is more for maintenance of sinus rhythm after conversion Of AF Used for VT orally(100-200 mg 6 hourly) less dose if CHF Iess GI side effect ; prominent vagolytic(urinary retention and dry mouth) Negative inotropic effect ;hypotension; torsades Pyridostgmine bromide or bethanecol may be used to reduse anticholinergic side Effects No digoxin interaction ;with class III cause torsades Class IB These drugs inhibit the fast Na current while shortening the action potential duration in a non diseased tissues Shortening of the repolarization period will ensure that QT prolongation does not occur These drug act selectively on diseased or ischemic tissue where they are thought to promote conduction block thereby interrupting reentry circuits Lidocaine has become standard IV agent for suppression of serious VT associated with AMI and with cardiac surgery The prophylaxis by lidocaine to prevent VT and VF in AMI is now outmoded Lidocaine is more effective in presence of high external K concentration therefore the hypokalemia must be corrected It has no value in treating SVT Side effect its generally free from hemodynamic side effect even in patient with CHF. The higher infusion rate of 3-4 mg / min may result in drowsiness Numbness, speech disturbances, and dizziness. Occasionally there is sinoaterial Arrest especially during coadminstration of other drugs that depress nodal function Drug interaction and combination Cimitidin, propranolol, or halothane will reduce hepatic clearance of lidocaine and Increase toxicity, while enzyme inducer the dose needed to be increased Beta blocker with lidocaine may produce bradyarrhythmia because beta blocker Reduce liver blood flow Phenytoin has 4 specific uses 1st in digitalis-toxic arrhythmias it maintains AV conduction especially in presence of Hypokalemia 2nd for VA occurring after congenital heart surgery 3rd for congenital prolonged QT syndrome when beta blocker has failed 4th in patient with epilepsy and arrhythmias Long half life permit once daily dosage with the risk serious side effect including Dysarthria, pulmonary infiltrate, and macrocytic anemia Phenytoin is hepatic enzyme inducer so alter the dose requirement of many drugs Including the antiarrhythmic (quinidine, lidocaine, and mexiletine) Mexilitine Like lidocaine is use for VA , unlikely lidocaine it can be given orally Advantage for VA Efficacy comparable to quinidine Little or no hemodynamic depression No QT prolongation No vagolytic effect But GI & CNS side effect limit the dose & possible therapeutic benefit Class IC They are potent ant arrhythmic used in control of paroxysmal SVT and VT resistant to other drug they have three major electrophysiological effect 1st powerful inhibitor of fast Na channel 2nd may variably prolong the action potential duration by delaying inactivation of slow Na channel 3rd inhibition of rapid repolarization action which may explain their marked inhibitory effect on his-purkinje conduction with QRS widening From 2nd & 3rd faster heart rate, increase sympathetic activity and diseased or ischemic myocardium we conclude the cause of proarrhythmia so these drugs must be avoided in patient with structural heart disease Flecainide Used for treatment of SVT & VT Its proarrhythmic effect limit its use especially in presence of structure heart disease because of poor LV function which predispose proarrhythmia It have negative inotropic effect Indication life threaten sustained VT Paroxysmal SVT including WPWS, & paroxysmal atrial flutter & fibrillation It contraindicated in patient with structure heart disease & in patient with right bundle Branch block & left anterior hemiblock (unless a pacemaker is implanted) Propafenone Is relatively safe in suppressing SV arrhythmias(WPW syndrome & recurrent atrial Fibrillation ) with no structural heart disease Has a potent membrane stabilizing activity & increase PR & QRS times without effect on the QT interval. It also has mild beta blocking & Ca antagonist properties Indication Life threatening ventricular arrhythmias & also SV arrhythmias There is strong evidence in use of propafenonoe in acute conversion of atrial fibrillation & maintenance of sinus rhythm it has GI side effect & proarrhythmia Relative C/I Preexisting sinus, AV or bundle branch or depressed LV function Patient with asthma Moricizine Is a phenothiazine derivative use for management of life threaten VA it has both class IB & class IC properties but it was ineffective as well as harmful Classification of antiarrhythmics (based on mechanisms of action) Class II – β–adrenergic blockers Have complex action including inhibition of spontaneous depolarization ( phase 4) & indirect Ca channel blocker which are less likely to be in the open state when not phosphorylated by the cyclic AMP Arguments of beta blocker The role of tachycardia in precipitating some arrhythmias The increase sympathetic activity in patient with sustained VT or AMI Role of 2nd messenger of beta adrenergic activity(CAMP) to cause ischemia related VF The associated antihypertensive & ant ischemic effect Indications Unwanted sinus tachycardia Paroxysmal atrial tachycardia due to emotion or exercise Exercise induced VA Arrhythmia of pheochromocytoma Heridatory prolong QT syndrome Arrhythmia of mitral valve Post MI arrhythmia Beta blocker include porpranolol, sotalol & acebutolol Acebutolol is attractive because of its cardioselectivity & its specific benefit in one Large post infarct survival trial Classification of antiarrhythmics (based on mechanisms of action) Class III – K+ channel blockers Developed because some patients negatively sensitive to Na channel blockers (they died!) Cause delay in repolarization and prolonged refractory period Includes Amiodarone – prolongs action potential by delaying K+ efflux but many other effects characteristic of other classes Ibutilide – slows inward movement of Na+ in addition to delaying K + influx. Bretylium – first developed to treat hypertension but found to also suppress ventricular fibrillation associated with myocardial infarction Dofetilide - prolongs action potential by delaying K+ efflux with no other effects Amiodarone Chiefly class III but with also powerful class I activity, class II & class IV Its established antiarrhythmic benefit & mortality reduction need to be balance against: Slow onset of action of oral therapy that require large loading dose Serious side effect Serious drug interaction that predispose to torsade de point Amiodarone also has; powerful class I effect Non competitively block alpha & beta receptor Weak Ca antagonist which explain bradycardia & AV nodal inhibition & low incidence of torsade de point Indications For recurrent VF of hemodynamically unstable VT Prophylactic control of life threatening VT especially post MI & CHF IV amiodarone is used for initiation of treatment & prophylaxis of ventricular Fibrillation or destabilizing VT but should monitor hypotension • Preventing reoccurrence of paroxysmal AF • Cardiac side effect • • • Amiodarone inhibit SA or AV node which can be serious in patient with prior Sinus node dysfunction or heart block In heart failure torsade de point rarely occur but we should avoid hypokalemia and digoxin toxicity • Pulmonary side effect • Pneumonitis leading to pulmonary fibrosis occurring in 10-17% at dose of 400 mg/day Thyroid side effect It contain iodine & similar to thyroxin structure, it inhibit peripheral conversion of T4 to T3 with main rise in T4 serum level but in most patient thyroid function is not Altered 6% of patient develop hypothyroidism 0.9% of patient develop hyperthyroidism Other side effect CNS side effect, proximal muscle weakness, peripheral neuropathy, neural symptom Testicular dysfunction, corneal microdeposite, photosensitivity Drug interaction Class IA antiarrhythmic, phenothiazine TCA, thiazide and sotalol will increase the Effect of amiodarone in prolonging QT interval It increase quinidine & procainamide level It increase phenytoin level It prolong prothrombin time & cause bleeding in patient on warfarin It increase plasma digitoxin concentration C/I: Severe sinus node dysfunction 2nd or 3rd degree heart block Cardiogenic shock Sever chronic lung disease Sotalol Its combine class II & class III properties, its active against: Sinus tachycardia, paroxysmal SVT, WPW arrhythmia with either antegrade or retroGrade conduction, recurrence of AF, ischemic VA & recurrent sustained VT or fibrillation Sotalol is used when amiodarone toxicity is feared but it is less active than amiodarone side effect: Are those of beta blocker (fatigue & bradycardia) also bronchospasm may be also Produced Pure class III agents: Ibutilide & Dofetilide Ibutilide is a methane sulfonamide derivative which prolong repolarization by inhibition of Delayed K current & by selective enhancement of the slow inward Na current It has no negative inotropic effect It used IV because of the 1st pass metabolism Its used in termination of AF & flutter with both single & repeated IV infusion Its effective as amiodarone in cardioversion of AF . Efficacy was higher in atrial flutter Than in AF ADVERSE AFFECT: QT interval prolongation & is dose dependent ,maximal at the end of infusion & return To base line within 2-4 hours following infusion Torsade de point is most significant adverse effect associated with it in about 4.3% occurring shortly after infusion period Dose 1mg over 10 min Dofetilide: Is a methane sulfonamide drug prolong action potential period & QT interval In a concentration related manner Its effect is by inhibition of the rapid component of the delayed K current It has mild negative chronotropic but not inotropic Its given orally Indication Cardioversion of persistent AF or atrial flutter to normal sinus rhythm Maintenance of sinus rhythm Dose : must be individualized by the calculated creatinin clearance & the QT prolongation Drug-interaction: Hepatic enzyme inhibitor will increase the level of it also drug as diuretic will add Prolongation of QT interval (due to hypokalemia ) Novel Class III Agents Azimilide Block both the slowly activating and rapidly activating components of the delayed rectifier K current, whereas sotalol,amiodarone,or dofetilide block only the rapidly activating component The advantage of blocking slow repolarization K current may be in condition of tachycardia and sympathetic stimulation when other blockers like sotalol are less likely to be effective Dronedarone amiodarone-like drug thought not to have noncardiac tissue side effect because it lacks iodine in its structure Classification of antiarrhythmics (based on mechanisms of action) Class IV – Ca2+ channel blockers slow rate of AV-conduction and increase refractory period of nodal tissue in patients with atrial fibrillation (slow the ventricular response rate in atrial arrhythmias) Terminate or prevent reentrant arrhythmias in which the circuit involves the AV node Now ,For termination of junctional tachycardias adenosine is the first choice Includes Verapamil – blocks Na+ channels in addition to Ca2+; also slows SA node in tachycardia Diltiazem Intravenous Magnesium Weakly blocks the calcium channel as well as inhibiting sodium and potassium channels It can be used to slow the ventricular rate in AF but is poor at terminating junctional tachycardias It may be agent of choice in torsade de pointes It can be used for refractory ventricular fibrillation but now superseded by IV amiodarone Adenosine It has multiple cellular effects including ; Opening of adenosine-sensitive inward repolarisation K channel to hyperpolarise with inhibition Of sinus & especially the AV node & indirectly to inhibit Ca channel opening Indications For paroxysmal narrow complex SVT ,usually AV nodal reentry or AV reentry such as in the WPW syndrome or in patients with a concealed accessory pathway. In wide-complex tachycardia of uncertain origin ,it can help the management by differentiating between VT or SVT . the latter case ,adenosine is likely to stop the tachycardia,whereas in VT there is unlikely to be any major adverse hemodynamic effect and the tachycardia continues Side effect & contraindications Headache (via vasodilation) ,provocation of chest pain ,flushing & excess sinus or AV nodal inhibition . the precipitation of bronchoconstriction in asthmatic patients can last for 30 min . Transsient new arrhythmias at the time of chemical cardioversion occur in about 65%. Because of a direct effect on atrial & ventricular myocardial refractoriness it has proarrhythmic effects including atrial and ventricular ectopy. contraindication Asthma ,second or third degree AV block,sick sinus syndrome Atrial flutter is a relative contraindication Digoxin Historically been the drug of choice for rate control in AF ,but its limitation must be recognized. The effects of digoxin are mediated by enhancement of vagal tone .it is less effective during state of high sympathetic tone as seen at the onset of an episode ,during exercise ,or in critically ill patient Digoxin is most effective as oral therapy in stable elderly patient who do not exercise vigorously or who may have underlying conduction disease or in combination with Ca channel blocker or B-blocker. References .Drug for the heart by Lionel H. Opie& Bernard J.Gersh .Basic & clinical pharmacology by Bertram G Katzung