* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Vision
Idiopathic intracranial hypertension wikipedia , lookup
Vision therapy wikipedia , lookup
Retinal waves wikipedia , lookup
Diabetic retinopathy wikipedia , lookup
Visual impairment wikipedia , lookup
Cataract surgery wikipedia , lookup
Macular degeneration wikipedia , lookup
Eyeglass prescription wikipedia , lookup
Visual impairment due to intracranial pressure wikipedia , lookup
Mitochondrial optic neuropathies wikipedia , lookup
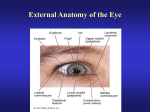
Vision Visible light is part of the electromagnetic spectrum Visible portion of electromagnetic spectrum (380 – 700 nm wavelength) has enough energy to make a reversible change in receptor molecules without permanently damaging them. Image formation on the retina Retinal image is inverted and reversed. Absence of receptors at optic disc creates blind spot in visual field about 15 degrees temporal to fixation point. Optical defects Myopic eye is too long, focal point is in front of retina. Correct with concave lens. Hyperopic eye is too short, focal point is behind retina. Correct with convex lens. Eye increases in size for 15 years. Refractive errors decease . Studies of experimental myopia in primates and chickens show retina controls growth of sclera and length of eye by detecting image blur. Retina can distinguish hyperopic blur from myopic blur though we can’t perceive difference. Diopter = 1/focal length in meters Relaxed eye ~ 60 D; Chambers, iris and lens Anterior and posterior chambers contain aqueous humor. Fundus of eye behind lens is filled with vitreous. Ciliary muscle + ciliary processes = ciliary body Aqueous humor is secreted into posterior chamber by highly vascularized folds, called ciliary processes, in secretory ciliary epithelium. Aqueous humor and intraocular pressure Aqueous humor is formed by ciliary processes and enters the anterior chamber through the pupil. Drains from the eye at the angle of the anterior chamber where it must pass through collection of tissue cords (trabecular meshwork) before entering canal of Schlemm. Intraocular pressure depends on the rate of aqueous production and the resistance to its outflow. Glaucoma Optic neuropathy in which optic nerve deteriorates with progressive enlargement and cupping of optic disc. There are several forms of glaucoma: Primary open angle glaucoma. 80% of all cases; afflicts 1% of people over 40; most common optic neuropathy among elderly. Often, but not always, accompanied by elevated intraocular pressure that can stop axoplasmic flow as nerve passes through sclera. Structural change in trabecular meshwork impedes aqueous outflow. Lowering IOP does not arrest disease. Programmed optic nerve death? Closed angle glaucoma. 10% of cases. Occurs when iris covers trabecular meshwork. Causes rise in IOP to > 40 mmHg that stops blood flow to optic nerve. IOP > 21 mmHg = ocular hypertensive Why is glaucomatous disk so cupped? • Most distinctive feature of glaucoma is deeping and enlargement of optic nerve cup. Occurs even when intraocular pressure (IOC) is normal. • Loss of large diameter axons (other optic neuropathies affect mainly small axons) • Collagen fibers of sclera form meshwork through with optic nerve must pass. In glaucoma this lamina cribosa bends backwards. Due to defective collagen? IOC? • Glia cells do not proliferate to fill gaps, resulting from dead axons, as they do in other inflammatory or ischemic optic neuropathies Accommodation and Presbyopia • • • Accommodation is the result of ciliary muscle contraction. When ciliary muscle is relaxed, zonular fibers are under tension and pull outward on lens, flattening it. Unaccommodated eye is focused on distant objects. When ciliary muscle contracts, it reduces pull of zonule fibers and lens becomes smaller and thicker. Due to elasticity of lens capsule. Change optical power 8 D. • Primary stimulus for accommodation is retinal image blur. Changes in image size and judgements of apparent distance can also act as stimulus. • Presbyopia: decreased accommodation amplitude with age. From 14 Diopters at 8 years to 1 D at 50 or 55 yr. No cure. Due to age related loss of elasticity. The lens and cataracts • • • • Lens is 90% protein, more than any other tissue in body. Transparency due to: 1) dense, uniform packing of crystallin protein molecules & 2) low water content maintained by ion pumping in epithelium. Lens is formed of concentric layers of long, thin fibers in an elastic capsule. Size of lens and number of fibers increase throughout life. Cataracts are opacities in lens. Can occur at any age but most age related. Cause nearly 50% of blindness worldwide. Multiple risk factors. Dominant cause is UV radiation which alters protein structure. Treat by surgical removal of lens. Aphakic eye severely hyperopic so usually insert plastic or silicone intraocular lens Optic disc, fovea and macula Optic disc Fovea Sclera Fovea OD choroid Fovea is fixation point in central retina and has highest acuity but low sensitivity. 1.5 mm diam. Occupies about 1 – 2 degrees visual space. At center of macula. Macula ~ 6 mm diam.. Has good acuity and occupies about 5 degrees of visual space. Age-related Macular Degeneration Progressive loss of central vision due to gradual degeneration of the photoreceptors. First symptom is usually blurring of central vision when reading. Usually affects both eyes. Most common cause of vision loss in people over 55. Causes of disease are unknown. Candidates include hereditary factors, cardiovascular disease, smoking, light exposure, nutrition—all may play a role. 10% AMD is exudative-neovascular (wet) form. Abnormal growth of blood vessels under macula leak blood into retina and damage photoreceptors. Rapid progression (months). Treat with laser therapy. 90% AMD non-exudative (dry) form. Gradual disappearance of retinal pigment epithelium over period of years. Loss of photoreceptors in affected areas. Patient usually retains some central vision. No treatment is available. Rod and cone outer segment Scotopic vision: High sensitivity; low spatial resolution. Starlight. Rods. Mesopic vision: rods and cones; Moonlight Photopic vision: Low sensitivity; high spatial resolution. Brighter than moonlight. Cones. Normal vision depends mostly on cones. Cones must capture 100 photons to produce response of 1 photo captured by a rod Visual pigment Phototransduction begins when a photon is absorbed by visual pigment in a receptor disk. Photopigment contains a light absorbing chromophore, retinal (an aldehyde of vitamin A) coupled with one of several proteins called opsin. Most studies on rods where photopigment is rhodopsin. Absorption of photon by rhodopsin molecule changes it from its 11-cis isomer to alltrans retinal. This triggers a sequence of biochemical events resulting in a receptor potential. Phototransduction involves closing cation ion channels in outer segment membrane 1. Absorption of photon converts 11-cis retinal to all-trans isomer 2. Activated rhodopsin stimulates G protein, Transducin 3. Activated G protein activates enzyme that breaks down cyclic GMP 4. Intracellular [cGMP] drops, plasma membrane channels close, Na+ can’t enter; cell hyperpolarizes. Amplifying cascade: 1 photon + 1 rhodopsin hydrolyze 250,000 cGMP per second Biochemical cascade and light adaptation Biochemical cascade initiated by photon capture greatly amplifies signal: Estimated that 1 light activated rhodopsin molecule can activate 800 transducin molecules. Each transducin molecule activates only 1 phosphodiesterase molecule but each of these may catalyze breakdown of up to 6 cGMP molecules. In this way absorption of one photon by a single rhodopsin molecule can cause about 200 ion channels to close and change the membrane potential about 1 mV. Light adaptation. Magnitude of amplification varies according to level of illumination. Photoreceptors are most sensitive in dim light, less sensitive in bright light. This prevents them from saturating and extends the range of light intensities over which they can operate. cGMP-gated channels in outer segment are permeable to Ca2+ as well as Na+. As illumination increases, more channels close and intracellular [Ca2+] drops. This decrease triggers changes in phototransduction cascade that reduce sensitivity of receptor to light. Additional factors include neural interactions between photoreceptors and horizontal cells. Structure of the Retina Direct path: Receptor – Bipolar cell – Ganglion cell. Indirect path: Receptor – Horizontal cell – Bipolar cell – Amacrine cell – Ganglion cell Horizontal and Amacrine cells mediate lateral interactions Retinal pigment epithelium contains melanin; prevents backscattering of light; essential role in renewing photopigments and phagocytosing photoreceptor disks that are sloughed off and regenerated. From Purves et al. 2004. Neuroscience Retinitis pigmentosa • • • Common hereditary retinopathy, affects 100,000 people in U.S. RP is group of hereditary eye disorders involving gradual degeneration of the photoreceptors. Main symptoms are night blindness, loss of peripheral vison, narrowing of retinal vessels and migration of pigment from disrupted retinal pigment epithelium into retina where it forms clumps near blood vessels. Progressive death of rods, may be followed by loss of cones Mutation may be X-linked or dominant or recessive autosomal gene. To date mutations identified on 30 genes. Many encode photoreceptor proteins. Pathogenesis is not well understood. Why do cones degenerate? Often protein that RP affects is not expressed in cones, e.g., rhodopsin. Dark clumps of pigment in retina Renewal of labeled amino acids in rods Normal phagocytosis of sloughedoff rod proteins by long processes of pigment epithelium Color vision: 3 classes of cones There are three types of cones with different photopigments that respond to Short, Medium or Long wavelengths. Individual cones are colorblind. Can’t discriminate changes in wavelength from changes in luminance. S M L Color perception depends on comparing activity of ganglion cells from different classes of cones. Color blindness • Trichromats (Normal) – Most people can match any color by adjusting the intensity of three superimposed light source producing S, M and L wavelengths About 8% of men and only 0.5% of women are color blind • Dichromats Can match colors with only two lights ; don’t see third color category – Protanopia– no red cones; x chromosome – Deuteranopia– no green cones; x chromosome – Tritanopia– no blue cones; chromosome 7 (rare) • Anomalous trichromats – Red or green gene is replaced by hybrid gene that has intermediate spectral sensitivity. (protanomalous if it is red gene; deuteranomalous if it is green gene that is abnormal) Central visual pathways Retinal projections: • • • Pretectal area of midbrain – Pupillary light reflex Superior colliculus – Saccadic eye movements – Multimodal maps of visual space Lateral geniculate nucleus – Cortical feedback, filters transmission to cortex – Part of pathway for conscious visual perception Most ganglion cells go to the lateral geniculate nucleus Axons from nasal half of each retina cross in optic chiasm. Each optic tract ‘looks’ at contralateral visual field Parvocellular layers Magnocellular layers Optic radiation to visual cortex Lateral geniculate n. Lateral ventricle LGN Meyer’s Loop Optic radiation Field of left eye Field of right eye In left cerebral hemisphere Calcarine sulcus Nolte p. 434 Left visual cortex contains right half of visual field of both eyes Seen only by right eye Inferior field Calcarine sulcus Field of left eye Fovea Superior field Field of right eye Nolte p. 436 Primary visual cortex is organized into columns Color sensitive regions RE LE Orientation columns Ocular dominance columns Visual Field Defects Visual field of Ophthalmic artery anurism Pituitary tumor Lesion in Myer’s Loop Visual deprivation and amblyopia • Amblyopia is diminished visual acuity due to failure to establish appropriate cortical connections early in life. • Strabismus (misalignment of eyes) can cause double vision and is most common cause of amblyopia. In some individuals brain suppresses input from one eye which may become effectively blind. Early surgical correction of extraocular muscle length important. • During development, axons of cells in the lateral geniculate compete for synaptic space on cells in visual cortex. This critical developmental period, when cortical synapses are being formed, lasts several years in children. Visual deprivation of one eye during part of the critical period (due to congential cateracts, or amblyopia caused by strabismus) can result in few cortical cells responding to the deprived eye which may become permanently functionally blind. • Visual deprivation after the end of the critical period when synapses for both eyes have been established does not affect vision. Temporal parvocellular pathway analyses form and color Lesions of the temporal vision-related cortical pathway cause inability to name and identify familiar objects, symbols, words, colors etc. For example: Prosopagnosia Prosopagnosia: inability to identify familiar faces Lesion of lingual, fusiform and parahippocampal gyri. Right side only or bilateral. Caused by stroke, tumor, demyelination or atrophy. Cannot recognize familiar face. Know face is a face and if sad or happy, but not whether they have seen it before. Cannot learn to recognize new face. Cannot distinguish between members of other classes of objects either. Bilateral inferior occiptiotemporal lesions Magnocellular parietal pathway analyzes motion Lesions of parietal vision-related cortex pathway cause visual illusions and deficits related to the distribution of visual attention and perception and manipulation of items in space. Examples: Hemispatial or unilateral neglect Akinetopsia Hemispatial or unilateral neglect Disorder of spatially directed attention caused by lesion of right posterior parietal region. Body centered instead of visual field centered. Self protraits after right posterior parietal lesion 2 mo 3.5 mo 6 mo 9 mo Akinetopsia: inability to detect motion Lesion in visual area V5 (MT) in parieto-occipital cortex which contains neurons sensitive to motion but not orientation or wavelength. Sees moving objects but does not perceive them as in motion.