* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Intro to Chemical Equations note
Process chemistry wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Water splitting wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Click chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Electrolysis of water wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Electrochemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Hydrogen atom wikipedia , lookup
Metalloprotein wikipedia , lookup
Transition state theory wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Atomic theory wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
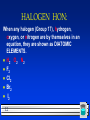
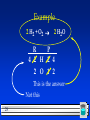
Chapter 4 Chemical Reactions 1 All chemical reactions have two parts: –Reactants - the substances you start with –Products- the substances you end up with The reactants turn into the products. Reactants Products 2 In a chemical reaction The way atoms are joined is changed Atoms aren’t created or destroyed. Can be described several ways: 1. In a sentence Copper reacts with chlorine to form copper (II) chloride. 2. In a word equation Copper + chlorine copper (II) chloride 3 Symbols in equations the arrow separates the reactants from the products Read “reacts to form” The plus sign means “and” (s) after the formula = solid (g) after the formula = gas (l) after the formula = liquid 4 Symbols used in equations (aq) after the formula - dissolved in water, an aqueous solution. 5 Symbols used in equations indicates a reversible reaction (more later) heat shows that , heat is supplied to the reaction Pt is used to indicate a catalyst is supplied, in this case, platinum. 6 What is a catalyst? A substance that speeds up a reaction, without being changed or used up by the reaction. 7 Skeleton Equation Uses formulas and symbols to describe a reaction doesn’t indicate how many. All chemical equations are sentences that describe reactions. 8 Convert these to equations Solid iron (III) sulfide reacts with gaseous hydrogen chloride to form iron (III) chloride and hydrogen sulfide gas. Fe2S3 (s) 9 + HCl (g) FeCl3 (s) + H2S (g) Nitric acid dissolved in water reacts with solid sodium carbonate to form liquid water and carbon dioxide gas and sodium nitrate dissolved in water. HNO3 (aq) + Na2CO3 (s) H2O (l) + CO2 (g) + NaNO3 (aq) 10 Now, read these: Fe(s) + O2(g) Fe2O3(s) Cu(s) + AgNO3(aq) Ag(s) + Cu(NO3)2(aq) 11 Pt NO2 (g) N2(g) + O2(g) HALOGEN HON: When any halogen (Group 17), hydrogen, oxygen, or nitrogen are by themselves in an equation, they are shown as DIATOMIC ELEMENTS. H2 O2 N2 F2 Cl2 Br2 I2 12 Balancing Chemical Equations 13 Balanced Equation Atoms can’t be created or destroyed All the atoms we start with we must end up with A balanced equation has the same number of each element on both sides of the equation. 14 C + O O O C O + O2 CO2 This equation is already balanced What if it isn’t? C 15 C + O O C O C + O2 CO We need one more oxygen in the products. Can’t change the formula, because it describes what it is (carbon monoxide in this example) 16 C Must + O O C O C O be used to make another CO But where did the other C come from? 17 C + C Must O O C O C O have started with two C 2 C + O2 2 CO 18 Where the numbers are mean different things….. 4Pb(NO3)2 19 3- only relates to the oxygen atom (called a “subscript”) 2-relates to everything inside the brackets(called a “subscript”) 4-relates to every atom in the compound (called the “coefficient”) Rules for balancing: Assemble, write the correct formulas for all the reactants and products Count the number of atoms of each type appearing on both sides Balance the elements one at a time by adding coefficients (the numbers in front) - save H and O until LAST! Check to make sure it is balanced. 20 Never change a subscript to balance an equation. – If you change the formula you are describing a different reaction. – H2O is a different compound than H2O2 Never put a coefficient in the middle of a formula – 2 NaCl is okay, Na2Cl is not. 21 Example H2 + O2 H2O Make a table to keep track of where you are at 22 Example H2 + O2 H2O R P 2 H 2 2 O 1 Need twice as much O in the product 23 Example H2 + O2 R P 2 H 2 2 O 1 Changes the O 24 2 H2O Example H2 + O2 2 H2O R P 2 H 2 2 O 1 2 Also changes the H 25 Example H2 + O2 2 H2O R P 2 H 2 4 2 O 1 2 Need twice as much H in the reactant 26 Example 2 H2 + O2 2 H2O R P 2 H 2 4 2 O 1 2 Recount 27 Example 2 H2 + O2 2 H2O R P 4 2 H 2 4 2 O 1 2 The equation is balanced, has the same number of each kind of atom on both sides 28 Example 2 H2 + O2 2 H2O R P 4 2 H 2 4 2 O 1 2 This is the answer Not this 29 Balancing Examples _AgNO3 + _Cu _Cu(NO3)2 + _Ag _Mg _P4 30 + _N2 _Mg3N2 + _O2 _P4O10 Odd Number Rule 31 If ,when balancing, there happens to be an odd number (other than 1) of an element on one side, and an even number on the other side, DOUBLE THE COEFFICIENT IN FRONT OF WHERE IT’S ODD, AND THEN START OVER (with that element)