* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 15
Survey
Document related concepts
Transcript
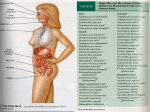
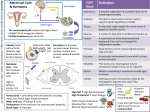
Chapter 15 Microbial Mechanisms of Pathogenicity Microbial Mechanisms of Pathogenicity • Pathogenicity: the ability to cause disease • Virulence: the degree or extent of pathogenicity • Disease is caused by the microbe overpowering the host’s defense (immune response) – Due to microbes directly damages host tissue – Due to accumulation of microbial waste products How Microorganisms Enter a Host • Portals of entry: the avenue by which a pathogen gains access to the body – Mucous membranes penetrate membrane lining; Most pathogens enter through the mucous membranes of the gastrointestinal and respiratory tracts • respiratory tract: by inhalation (easiest and most commonly used route) • gastrointestinal tract: by food, water and contaminated fingers • genitourinary tract: sexually • conjunctiva: birth canal, contact lenses, hands/fingers How Microorganisms Enter a Host – Skin through hair follicles and sweat gland ducts; intact skin is the first line of defense; most microorganisms cannot penetrate intact skin – Parenteral route: direct deposition into tissues beneath the skin and mucous membranes by punctures, injections, bites, cuts, wounds, surgery and splitting • Many pathogens have a preferred portal of entry entry through other portal may not cause disease Numbers of invading microbes • ID50: Infectious dose for 50% of the test population – Used to compare the relative virulence of a microbe under experimental conditions – e.g. Bacillus anthracis (can cause infection via 3 different portals of entry Portal of entry Skin (cutaneous anthrax) ID50 10-50 endospores Inhalation (inhalation anthrax) 10,000-20,000 endospores Ingestion (gastrointestinal anthrax) 250,000-1,000,000 endospores Numbers of invading microbes • LD50: Lethal dose (of a toxin) for 50% of the test population – Used to express the potency of a toxin – e.g. LD50 in mice botulinum toxin 0.03 ng/kg Shiga toxin 250 ng/kg staphylococcal enterotoxin 1,350 ng/kg Adherence (Attachment) • Adhesins/ligands bind to complementary surface receptors on host cells • Host cell receptors are typically sugars (e.g. mannose) • Majority of adhesins on the microorganisms are glycoproteins or lipoproteins – Glycocalyx – Fimbriae – M protein Streptococcus mutans Escherichia coli Streptococcus pyogenes Adherence (Attachment) – Opa protein – Tapered end Neisseria gonorrhoeae Treponema pallidum • Biofilms: a microbial community that usually forms a slime layer on a surface – Mass of microbes attach to living and nonliving surfaces by their extracellular products (e.g. glycocalyx) – e.g. Dental plaque, scum on shower doors, contact lenses, heart valves, medical catheters – Resist disinfectants and antibiotics How Bacterial Pathogens Penetrate Host Defenses • Capsule increases the virulence by resisting the phagocytosis – e.g. Streptococcus pneumoniae, Klebsiella pneumoniae, Haemophilus influenzae, and Bacillus anthracis – Host’s defense: antibodies produced against capsules allows encapsulated bacteria to be destroyed by phagocytosis How Bacterial Pathogens Penetrate Host Defenses • M proteins (on cell surface and fimbriae) mediates attachment and helps resist phagocytosis – Host’s defense: antibodies against M proteins • Waxes in the cell wall of Mycobacterium tuberculosis resist digestion by phagocytes; bacteria can even multiply inside phagocytes How Bacterial Pathogens Penetrate Host Defenses • Extracellular (exoenzymes) enzymes aid virulence – Coagulase coagulate (clot) fibrinogen in blood; fibrin clot may protect the bacterium from phagocytosis and isolate bacteria from other defenses of the host – Kinases digest/dissolve fibrin clots; allows bacteria to spread from a localized area How Bacterial Pathogens Penetrate Host Defenses – Hyaluronidase hydrolyses hyaluronic acid; cause the tissue blackening of infected wounds and allows bacteria to spread from the initial site of infection – Collagenase hydrolyzes collagen; helps bacteria to spread – IgA proteases destroy IgA antibodies; remove protection on mucosal surfaces How Bacterial Pathogens Penetrate Host Defenses • Antigenic variation: alter surface proteins (antigens) to escape antibodies (can inactivate or destroy antigens) – activating alternative genes (e.g. Opa protein) • Penetration into the host cell cytoskeleton – Use host’s cytoskeleton (actin) to move through the host cytoplasm and between host cells (e.g. Shigella species and Listeria species) – Invasins: cause formation of an actin basket around Salmonella and move bacteria into the cell Penetration into the Host Cell Figure 15.2 How Bacterial Pathogens Damage Host Cells • If phagocytes can eliminate invading microorganisms, no further damage is done. • If pathogens overcome the host defense damage to the host cells – Use the host’s nutrients – Cause direct damage at the site of infection – Produce toxins (cause damage in other parts of the body) – Induce hypersensitivity reactions Using the host’s nutrient • Iron (required for most pathogens’ growth) – Some produce siderophores secreted by bacteria to take iron from host iron-transport proteins – Some have receptors that bind directly to irontransport proteins and hemoglobin – Some produce toxins kill the host cells to obtain iron from dead host cells Direct damage • Pathogens cause damage by rupturing the host cells as they metabolize and multiply inside cells – Use host cell for nutrients and produce waste – As the host cells rupture, pathogens are released and free to spread infections • Most damage is done by production of toxins Production of toxins • Toxin: poisonous substances that contribute to pathogenicity – Exotoxins (many are encoded by plasmids or lysogenic conversion) and endotoxins • Toxigenicity: ability to produce a toxin • Toxemia : presence of toxin in the host's blood • Toxoid: inactivated toxin used in a vaccine (e.g. diphtheria and tetanus vaccine) • Antitoxin: antibodies against a specific toxin; provide immunity to exotoxins Exotoxins • Proteins (many are enzymes can be used over and over again) • Most of the genes are carried on bacterial plasmids or phages • Soluble in body fluids rapidly diffuse and transport throughout the body Figure 15.4a Exotoxin • Only need minute amount to cause diseases; among the most lethal substances known – Signs and symptoms of the disease is caused by exotoxin, not by bacteria • Can induce formation of antitoxins • Three principal types of exotoxins (based on structure and function) – A-B toxins or type III toxins – Membrane-disrupting toxins or type II toxins – superantigens or type I toxins Exotoxins • A-B toxins (type III toxins) – Majority of exotoxins in this category – 2 parts: A is the active (enzyme) component & B is the binding component Figure 15.5 Exotoxins • Membrane-disrupting toxins or type II toxins – Lyse host’s cells by: • Making protein channels in the plasma membrane (e.g. hemolysins and leukocidins) – hemolysins: form protein channels to destroy erythrocyte (staphylococci and streptococci) • Disrupting phospholipid bilayer – Contribute to virulence by killing host immune cells (phagocytic cells) and aid bacteria to escape from phagosomes into the host’s cytoplasm • e.g. Leukocidins (kill phagocytic leukocytes and macrophages by forming protein channels) Exotoxins • Superantigens or type I toxins – Bacterial proteins (e.g. staphylococcus toxins which cause food poisoning & toxic shock syndrome) – Cause an intense immune response to release cytokines from host T cells (nonspecific stimulation of T cells causing proliferation and release massive amount of cytokines) – Excessively high levels of cytokines (small protein hormones) enter the bloodstream to cause fever, nausea, vomiting, diarrhea, shock, and even death Exotoxins • Neurotoxins: attaches to nerve cells and interferes with normal nerve impulse conduction • Cytotoxins: attaches to wide variety of cells and either kills host cells or alters their function – Erythrogenic toxins: damage the plasma membrane of blood capillaries under the skin and produce a red skin rash (e.g. Streptococcus pyogenes) • Enterotoxins: attaches to the lining of the gastrointestinal tract and cause gastroenteritis Exotoxins Exotoxin Lysogenic conversion A-B toxin. Inhibits protein synthesis. + • Streptococcus pyogenes Membrane-disrupting. Erythrogenic. + • Clostridium botulinum A-B toxin. Neurotoxin + • C. tetani A-B toxin. Neurotoxin • Vibrio cholerae A-B toxin. Enterotoxin • Corynebacterium diphtheriae • Staphylococcus aureus Superantigen. Enterotoxin. + Endotoxin = Lipid A Figure 15.4b Bacterial Cell Wall Fig. 4.13 b & c Endotoxins • Lipopolysaccharides (Lipid A portion) • Released when gram-negative bacteria die and their cell walls lyse – Antibiotics can lyse the bacterial cells to release endotoxins – Endotoxins also released during bacterial replication • Salmonella typhi, Proteus spp., and Neisseria miningitidis Endotoxins • All endotoxins produce the same sings and symptoms; however not to the same degree – – – – Chills, fever, weakness, generalized aches In some cases, shock and even death Can also cause miscarriage Activate blood-clotting proteins cut off or reduce blood supply death of tissues = disseminated intravascular clotting • Stimulate macrophages to release cytokines in very high concentrations (toxic level) – IL-1 and TNF Endotoxins • Fever (pyrogenic response) Figure 15.6 – Aspiring & acetaminophen reduce fever by blocking the synthesis of prostaglandins Endotoxins • Shock: any life-threatening loss of blood pressure – Septic shock: shock caused by bacteria – Endotoxic shock: shock caused by gram-negatives • Release of tumor necrosis factor (TNF) or cachectin by phagocytes (after ingesting gramnegative bacteria) into the bloodstream TNF damage blood capillaries drop in blood pressure septic shock Endotoxins • Do not induce effective antitoxins formation – Antibodies are produced, but tend not to counter the effect of the toxin • Endotoxins are more stable at high heat than exotoxins • Limulus amoebocyte lysate (LAL) assay used to detect presence of endotoxin in drugs, medical devices, and body fluids Plasmids, lysogeny, and pathogenicity • Plasmids carry genes for antibiotic resistance (R factors) & information for pathogenicity (virulence factors) – Encode for toxins, capsules, and fimbriae • Prophage (lysogeny) lysogenic conversion – Immune to infection by the same type of phage – Increase pathogenicity (genes for toxins, capsules) – Increase number of pathogenic bacteria (horizontal transfer) Pathogenic Properties of Viruses • Mechanism for evading host defenses – Penetrate and grow inside host cells to evade the reach of components of the immune system – Gain access to the potential host cells using attachment sites (on virus) specific to receptors on the potential host cells • Sometimes, attachment sites mimic substances useful to the host cells – Hide attachment sites from the immune response + attack components of the immune system directly (e.g. HIV virus) Pathogenic Properties of Viruses • Cytopathic effects (CPE) of viruses: visible effects of viral infection – Cytocidal effects: result in cell death – Noncytocidal effects: result in cell damage but not cell death – Stop synthesis of macromolecules in the host; stop mitosis; loss of contact inhibition – Formation of inclusion bodies; syncytium – Induce production of interferons in infected cells; antigenic changes on the surface of the infected cells; chromosomal changes in the infected cells; transformation Cytopathic Effects of Viruses Table 15.4 Pathogenic Properties of Fungi • No well-defined set of virulence factors • Fungal waste products (toxins) may cause symptoms – Tichothecene toxins inhibit protein synthesis (e.g. Fusarium) – Ergot toxin = hallucinogen (e.g. Claviceps) – Aflatoxin (e.g. Aspergillus) is carcinogenic – Mycotoxins (neurotoxins such as phalloidin & amanitin, produced by Amanita) may be lethal if ingested Pathogenic Properties of Fungi • Chronic infections provoke an allergic response • Some have Virulence factors such as proteases (e.g. Candida, Trichophyton) – Allow attachment of the fungi by modifying host cell membranes • Capsule prevents phagocytosis (e.g. Cryptococcus) • Decrease synthesis of receptors for antifungal drugs & become resistant to the drugs Pathogenic Properties of Protozoa • Presence of protozoa & protozoan waste products may cause symptoms • Avoid host defenses by – Growing in phagocytes (e.g. Plasmodium & Toxoplasma) – Antigenic variation (e.g. Trypanosoma) • Attach and digest the host cells and tissue fluids (e.g. Giardia) Pathogenic Properties of Helminths and Algae • Presence of parasite (helminth) interferes with host function • Parasite's metabolic waste can cause symptoms • Use host tissues for their growth cellular damage (cause symptoms) • Few species of algae (dinoflagellates) produce neurotoxins – e.g. Saxitoxin cause paralytic shellfish poisoning Portals of Exit • Microbes leave the body through specific routes to spread infections generally a microbe uses the same portal for entry and exit – Respiratory tract (most common portal of exit) via mouth and nose by coughing or sneezing – Gastrointestinal tract (most common portal of exit) via feces or saliva – Genitourinary tract via secretions from the penis and vagina (for sexually transmitted diseases) or urine Portals of Exit – Skin or wound infections via shedding, contact, or drainage from wounds – Infected blood via biting arthropods, needles/syringes Mechanisms of Pathogenicity Figure 15.9 Chapter Review Figure 15.9 Chapter Review 1. Know different portals of entry and exit, and how they enter or leave a host – Generally a microbe uses the same portal for entry and exit • – respiratory and gastrointestinal tracts = most common portal of entry and exit Mucous membranes • • • respiratory tract – enter by inhalation; exit by mouth and nose through coughing or sneezing gastrointestinal tract – enter by food, water, and contaminated fingers; exit by feces or saliva genitourinary tract – transmitted sexually; exit through secretions from the penis and vagina (for STDs) or urine Chapter Review – Skin cannot penetrate unbroken skin; enter through hair follicles and sweat gland ducts; exit through shedding, contact (e.g. sweat) – Parenteral route: direct deposition into tissues beneath the skin and mucous membranes by punctures, injections, bites, cuts, wounds, surgery and splitting; if there is wound infection, exit through drainage from wounds, blood-borne pathogen exit through biting arthropods or needles/syringes – Many pathogens have a preferred portal of entry; if enter through different portal, may not cause disease Chapter Review 2. Know how pathogens adhere or attach to a susceptible host – Pathogens use adhesins or ligands to bind to complementary surface receptors on host cells – E.g. of adhesins/ligands: glycocalyx (Streptococcus mutans); fimbriae (Escherichia coli); M protein (Streptococcus pyogenes); Opa protein (Neisseria gonorrhoeae) – Pathogens can also use biofilms to adhere (mass of bacteria secrete glycocalyx) to living and nonliving surface; biofilms resist disinfectants and antibiotics Chapter Review • • Biofilms: a microbial community that usually forms a slime layer on a surface e.g. Dental plaque, scum on shower doors, contact lenses, heart valves, medical catheters 3. Know how pathogens penetrate or evade host defenses – Capsule formation: resist phagocytosys host antibodies produced against capsules allow encapsulated bacteria to be destroyed by phagocytosis Chapter Review – Cell wall components (contain chemical substances) contribute to virulence • M proteins: mediates attachment and helps resist phagocytosis antibodies produced against M protein by host allows bacteria to be destroyed • Waxes in the cell wall of Mycobacterium: resist digestion by phagocytes; bacteria can even multiply inside phagocytes tend to become a chronic disease – Extracellular enzymes (exoenzymes) aid in virulence • protect the bacterium by forming a protective clot to avoid phagocytosis and other host’s defenses Coagulase Chapter Review • Allows bacterium to spread from the initial site of infection kinases, hyaluronidase & collagenase • Remove protection on mucosal surfaces IgA proteases – Antigenic variation: escape inactivation or destruction by host’s antibodies – Penetration into the host cell cytoskeleton • Invasins (Salmonella bacteria) to carry bacteria into the cell • Use host’s cytoskeleton to move through the host cytoplasm and between host cells (Shigella species and Listeria species) Chapter Review 4. Know how bacteria pathogens can damage host cells – Cause direct damage at the site of infection by rupturing the host cells as they metabolize and multiply Release from the host allows spread of infections – Production of toxins cause the most damage to a host (cause damage in other parts of the body) • Many of the exotoxins (proteins; many are enzymes) are encoded by plasmids or lysogenic conversion • Endotoxins are part of gram-negative cell wall (Lipid A) Chapter Review – Carrying plasmids allow bacteria to become antibiotic resistant; increase pathogenicity (carry virulence factors such as genes for toxins, capsule, and fimbriae) – Lysogenic conversion: prophage carry genes for toxins & capsules to increase pathogenicity; increase number of pathogenic bacteria (horizontal transfer) Chapter Review 5. Know pathogenic properties of viruses – Viruses can cause cytocidal effects (kill host cells) or noncytocidal effects (result in cell damage, not cell death) – Mechanisms for evading host defenses • Evade the host’s immune response by growing intracellularly • Gain access to the host cells using attachment sites (on virus) to bind to specific receptors (on hosts) • Hide attachment sites (on virus) from the immune response or attack components of the immune system directly Chapter Review 6. Know pathogenic properties of fungi, protozoa, helminths, and algae – fungi toxins (e.g. aflatoxin & mycotoxins); capsules; chronic infections cause allergic response; some become resistant to antifungal drugs by decreasing synthesis of receptors for the drugs – Protozoa avoid host defense by growing inside phagocytes; antigenic variation; damage to host tissues; metabolic waste Chapter Review – Helminth metabolic waste; cellular damage (tissue damage) – Algae (dinoflagellates) neurotoxins 7. Know these terms: pathogenicity, virulence, toxigenicity, toxoid, antitoxin, septic shock