* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Zoonosis
Bacterial morphological plasticity wikipedia , lookup
Infection control wikipedia , lookup
Plasmodium falciparum wikipedia , lookup
Hepatitis B wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Gastroenteritis wikipedia , lookup
Neonatal infection wikipedia , lookup
Neisseria meningitidis wikipedia , lookup
Anthrax vaccine adsorbed wikipedia , lookup
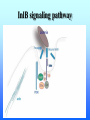
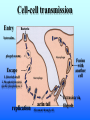
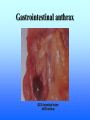
Listeria monocytogenes Bacillus Zoonosis Erysipelothrix Listeria monocytogenes http://textbookofbacteriology.net/Listeria.html Listeria 1. Overview 2. Bacteriological identification 3. Sources of Listeriosis 4. Pathogenic Mechanisms 5. Treatment Overview • 3 categores of listeriosis – Adult Disease – Disease in pregnant women – Fetal Disease • 1 Human pathogenic species – L. monocytogenes L. monocytogenes – Gram positive coccobacilli in pairs or short chains – Facultative anaerobe – Facultative intracellular pathogen – Motile (end-to-end tumbling) at 250 C not at 370 C – Weakly β-hemolytic on sheep blood agar – Can grow and replicate at 40 C and in high salt concentration. Sources of listeriosis • Humans can be intestinal carriers (1-5%) • Found in a feces of mammalian, avian, fish and crustacean species • Isolated from soil, silage, and other environmental sources • Food sources raw vegetables, unpasteurized milk, fresh soft cheese, and meats • Nosocomial transmission (neonatal nurses) Susceptible Populations • Elderly and Immunocompromised • Pregnant women • Neonates Listeriosis in the Normal Adult Soil Food Ingestion Large Intestine Liver & Spleen Immune Response Small Intestine Recovery Listeriosis in Normal Adult • Acute febrile gastroenteritis • Symptoms included body aches, fever, headache, diarrhea, and vomiting • Illness 24 h after ingestion of bacteria and usually lasts 2 days. Common symptoms include fever, watery diarrhea, nausea, headache, and pains in joints and muscles. • L. monocytogenes should be considered to be a possible etiology in outbreaks of febrile gastroenteritis when routine cultures fail to yield a pathogen. Listeriosis Elderly & Immuncompromised Death Large Intestine (gastroenteritis) Brain (meningitis) Blood Small Intestine Liver & Spleen Listeriosis in the fetus Abortion Sepsis at birth Mother Granulomatosis infantiseptica Fetus Placenta Liver & Spleen Blood Ingestion Small Intestine Clinical Case A 38-year-old woman was at her 31st week of gestation. Fever and chills for 3 days. No history of respiratory, urinary or gastrointestinal tract infection. Chills and general malaise 3 days prior to this admission. Decreased fetal movement and fetal tachycardia were noted. On admission, leukocytosis. Rupture of membrane with meconium stain 12 h after admission. 14 hours after admission, the fetal heart rate suddenly decelerated. Decreased fetal movement was also noted. A male infant in fetal distress was delivered by caesarean section 15 h after admission. Infant was transferred to the neonatal ICU for possible neonatal sepsis. Seizure developed. Cerebrospinal fluid (CSF) was yellow and turbid. Treatment of neonatal meningitis. Blood cultures and CSF culture yielded the same organism. Listeriosis in the Neonate 5 days – 3 weeks post delivery Purulent meningitis OR Meningo-encephalitis with sepsis < 5 days post delivery Sepsis Meningitis early onset Mother late onset Ingestion Neonate Small Intestine Large Intestine Pathogenesis • Internalins – InlA is responsible for uptake into epithelial cells and is required for crossing the intestinal barrier – InlB mediates entry into a variety of cell types but does not contribute to crossing of the intestinal cell barrier – Both InlA and InlB are needed for traversal of the fetoplacental barrier InlA signaling pathway Listeria InlA E-cadherin Plasma Membrane catenins β α InlB signaling pathway Pathogenesis • Phagolysosome - low pH activates exotoxin • β-hemolysin (listeriolysin O- exotoxin) – Cholesterol-dependent cytolysin – Forms a pore in the phagolysosome – Only present in virulent strain Pathogenesis • Phospholipase C (2 types) • Phosphatidylinositol-specific phospholipase C • Phosphatidylcholine phospholipase C- broad-range phospholipase • Both enzymes act in synergy with listeriolysin O to lyse the phagolysosome. Cell-cell transmission Entry Bacteria Internalins phagolysosome Fusion with another cell Escape 1. listeriolysin O 2. Phosphatidylinositolspecific phospholipase C replication actin tail Movement through cell Extrusion via filopods Treatment • Penicillin or ampicillin alone or in combination with gentamicin Overview • Infection is occupationally related – – – Fishermen Butcher Veterinarians • Contact with animals, their products or wastes • Three types of human infection: • Localized cutaneous • Generalized cutaneous • Septicemic which is associated with endocarditis Bacteriological identification • Gram positive • Microaerophillic bacillus • Thin, pleomorphic • Non-motile • Non-encapsulated • Non-sporulating Morphology/Physiology • Grows well on most laboratory media. • After 48 hours colonies are small to pinpoint. • Colonies are non-pigmented and transparent. • The α-hemolysis will develop after two days Sources of Erysipeloid • Acquired through skin abrasions • Inflammatory violaceous lesion at the infection site (usually fingers or hand) • Lesion is pruritic and painful • Lesion is non-supporative Pathogenic Mechanisms • Hyaluronidase • Neuraminidase Diagnosis and treatment • Diagnosis • History • Culture • Treatment • Penicillin • Resistant to vacomycin Bacillus anthracis: Overview CDC A photomicrograph of Bacillus anthracis bacteria using Gram stain technique. Anthrax : Overview • Gram-positive rods which form spores. • Transmission by contact with infected animals or contaminated animal products. • Primarily disease of cattle, sheep and goats. • No person-to-person transmission of inhalation anthrax. Anthrax : Overview • 3 distinct clinical presentations – Cutaneous – Gastrointestinal – Pulmonary Bacterial identification of B. anthracis Culture Characteristics • Incubated at 35-37o C • Cultures examined within 18-24 hours of incubation • Growth may be observed as early as 8 hours after inoculation. • Grows well on Sheep Blood Agar, but does not grow on MacConkey agar. Colony Characteristics • At 15-24 hours well isolated colonies are 2 - 5 mm. • Non-hemolytic, nonpigmented. Sheep blood agar plate culture of Bacillus anthracis and Bacillus cereus. CDC/Dr. James Feeley In contrast to colonies of B. cereus and B. thuringiensis, colonies of B. anthracis are not β-hemolytic. Microscopic Characteristics • Vegetative cells are in short chains of 2-4 cells that are encapsulated. • Large gram-positive rod • The capsule can be visualized microscopically using India Ink. • Spores are not generally present in clinical material; CO2 levels within the body inhibit sporulation. • Spores may be seen in material from wound eschars, but would not be seen in body fluids. India Ink Staining of Clinical Samples (Blood and CSF) for Capsule • India ink for visualization of encapsulated B. anthracis in clinical samples. • The capsule will appear as a well-defined clear zone around the cells for the positive control. CDC/Courtesy of Larry Stauffer, Oregon State Public Health Laboratory Isolation From Clinical Specimens • Blood specimens – There may be enough organisms in the blood to see them on direct smears by gram stain. – B. anthracis appears as short chains of 2-4 cells which maybe encapsulated. • Swab specimens for cutaneous Anthrax. • Sputum specimens • CSF specimens. Pathogenic Mechanisms • Exotoxins – plasmid-encoded (pXO1) – 3 components • Protective Antigen • Edema Factor • Lethal Factor • Capsule – Plasmid encoded (pXO2) – Poly D-glutamic acid Exotoxins • Protective antigen – Forms a pore in the endosome allowing EF and LF to enter the cytosol of the cell. • Edema Factor – calmodulin-dependent adenylate cyclase • Lethal factor – a zinc-dependent endopeptidase specific for mitogenactivated protein kinase kinase (MAPKK) Clinical Anthrax • Cutaneous • Gastrointestinal • Inhalational Cutaneous • Most common form (95%) • Inoculation of spores under skin • Incubation: hours to 7 days • Small, itchy, raised area on the skin ulcer surrounded by blister like lesions (24-28h) • Blister ruptures to form a painless ulcer (eschar) with a characteristic black necrotic center • Profound edema around lesions • Death 20% untreated; rare if treated Cutaneous Anthrax Day 5 Cutaneous Anthrax CDC:27 year old white female with cutaneous anthrax on right forearm Cutaneous Anthrax Day 7 Cutaneous Anthrax Day 12 Gastrointestinal • Ingestion of contaminated meat • Incubation: hours or up to 7 days • Fever, acute gastroenteritis, vomiting blood, bloody diarrhea • Mortality rate 25 -60% Gastrointestinal anthrax CDC: Intestinal lesion of GI anthrax Inhalational • Inhalation of spores • Most likely form of bioterrorist attack. • Incubation: 1 to 6 days, but can be as long as 43 days. • Gram stain of the blood and blood culture, but not until late in the course of the illness. • Only vegetative encapsulated bacilli are present during infection, spores are not found in the blood. Inhalational • Gradual onset (1-6 days) – fever, non-productive cough, muscle pain, malaise • Short period of improvement • Abrupt development of severe respiratory distress – dyspnea – diaphoresis – stridor – cyanosis – Shock and death usually occur within 24-36 h after the onset of respiratory distress • Mortality rate ~100% despite aggressive treatment Anthrax Treatment • Most B. anthracis strains are sensitive to a broad range of antibiotics. – Penicillin, ciprofloxacin, or doxycycline • Treatment should be initiated early. Post-Exposure Treatment • Start oral antibiotics as soon as possible. • Antibiotics for 60 days. Anthrax: Vaccine • Current U.S. vaccine (FDA Licensed) – FDA approved for persons 18-65 year of age – 93% protective against cutaneous and may also be protective against inhalation anthrax. – FDA approved for 6 dose regimen over 18 months with yearly boosters – 3 dose regimen (0, 2, and 4 weeks) may be effective for post-exposure treatment Anthrax: Vaccine – The vaccine is a cell-free filtrate that contains protective antigen and alum. – Pregnant women should not be vaccinated. – Limited availability