* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Neonatal infection wikipedia , lookup
Epidemiology of syphilis wikipedia , lookup
Infection control wikipedia , lookup
Urinary tract infection wikipedia , lookup
Hepatitis B wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Germ theory of disease wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Typhoid fever wikipedia , lookup
Gastroenteritis wikipedia , lookup
Globalization and disease wikipedia , lookup
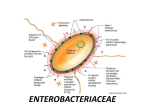
Gram-Negative Rods General Classification • Based on source or site of infection 1. Enteric tract 2. Respiratory tract 3. Animal sources Source or site of infection • Enteric tract Both within and outside: Escherichia, Salmonella Primarily within: Shigella, vibrio, Campylobacter Outside only: Klebsiella-Enterobacter-Serratia group, Proteus-Providencia-Morganella group, Pseudomonas, Bacteroides Source or site of infection (continued) • Respiratory tract: Haemophilus, Legionella, Bordetella • Animal sources: Brucella, Francisella, Pasteurella, Yersinia Classification Based on morphology, biochemical traits and genetic (phylogenetic) relationship • • • • Enterobacteriaceae Pseudomonaseae Vibrionaceae Cocobacilli Enterobacteriaceae • A heterogenous family • Mostly found in colon of human and other animals • Different pathogenetic mechanisms • Facultative anaerobic • Glucose fermentation • None have cytochrome oxidase • Reduce nitrates to nitrites Most important genuses in Enterobacteriaceae family • • • • • • • • Escherichia Shigella Salmonella Klebsiella Enterobacter Serratia Proteus Yersinia In contrast to Enterobacteriaceae • Pseudomonas Gram negative rods: • Non-fermenting (Strict aerobic) • Not reduce nitrate • Oxidase-positive General structure of cells in Enterobacteriaceae • All have Endotoxin • Some have Exotoxins, mostly called enterotoxins • Three surface antigens: O antigen: outer polysaccharide portion of the lipopolysaccharide (repeating 3-4 oligosaccharide sugars 15-20 times). A basis for the serologic typing (about 2000 types of Salmonella and 150 types of E. coli). H antigen: on the flagellar protein (in E.coli and Salmonella and not in Klebsiella and shigella). Unusual H antigens in Salmonella called phase 1 and phase 2. The organism can reversibly change in antigenicity to evade the immune response. K polysaccharide antigen: In encapsulated organisms such as Klebsiella. Identified by quellung (capsular swelling) reaction in the presence of specific antisera used for epidemiologic purposes. In S. typhi, it is called Vi (or virulence) antigen. quellung (capsular swelling) reaction Laboratory diagnosis • Culture for isolation Suspected specimens are inoculated onto 2 media: 1. Blood Agar 2. A selective differential medium (MacConkey’s agar or eosin-methylene blue, EMB agar. The differential ability is based on lactose fermentation as the most important criterion in identification of these organism. Non lactose fermenters form colorless colonies. Selective effect is exerted by bile salts or bacteriostatic dyes Laboratory diagnosis (continued) • Culture for identification • Screening biochemical tests for a final definitive identification: Triple sugar Iron Agar (TSI) Almost enough to identify the genus but array of 20 or more biochemical tests to identify the species. Triple Sugar Iron Agar and Urea Agar Triple Sugar Iron Agar • Indicator : Phenol red. • Components: - Iron or Ferrous sulfate - 3 sugars: glucose, lactose, and sucrose • Black FeS indicates the production of SH2 (Ferrous sulfate + Solfate reductase ferro sulphide (Sfe) Different observations for TSI Reactions Slant Button Gas H2S Representative genera Acid Acid + - Escherichia, Enterobacter, Klebsiella Alkaline Acid - - Shigella, Serratia Alkalin Acid + + Salmonella, Proteus Alkalin Alkalin - - Pseudomonas Urea Agar Indicator: Phenol red. Component : Urea If the bacterium Produces urease: Urea is hydrolyzed to NH3 and CO2 ---> light orange changes to reddish purple (in Proteus and K pneumoniae Amonium citrate (Simmons Citrate) Indicator: Bromothymol blue If the bacterium can utilizes ammonium dihydrogen phosphate (a salt of ammonium) and sodium citrate as sole source of nitrogen and carbon, the indicator turns to blue at alkalin pH due to releasing ammonia. Motility • SIM medium: SH2, Indole, Motility • Proteus: Swarms • Differentiation between Enterobacter cloacae (motile) from Klebsiella pneumoniae (Non motile) Indole • Tryptophan Tryptophanase Deamination Intermediate products: Indole + …. • Detection: 5 drops Kovac’s reagent (contains paradimethlaminobenzaldehyde = PDAB) is added Red ring Serology Usually in Salmonella, Shigella and E.coli final detection (serotyping) by agglutination Ag+Ab test. Coliforms • That part of this family which are normal inhabitants of the colon: E. coli Enterobacter Klebsiella Citrobacter So, E.coli is the indicator for fecal contamination of water supply: Lactose fermentation, Acid and gas production, growth at 44.5 C and typical colony on EMB. 4 colony count per dL in drinking water is indicative of unacceptable fecal contamination. Antibiotic therapy • Must be individually tailored to the antibiotic sensitivity test (Antibiogram). • Penicillin and cephalosporin families. Aminoglycosides (Gentamicin, amikacin, kanamycin, streptomycin …), Chloramphenicol, tetracyclines, quinolones and sulfonamides. Escherichia coli • Diseases 1. Diarrhea or dysentery 2. UTI 2. The most common cause of sepsis among negative rods 3. One of the 2 important causes of neonatal meningititis (the other is the group B streptococci) due to colonization of vagina by these organisms in about 25% of pregnant women. Virolence factors: • • • • Pili Capsule Endotoxin Two exotoxins (enterotoxins). E. coli pili (fimbriae) mannose galactose – glycolipids – glycoproteins 25 Pathogenesis • E. coli attaches to the surface of jejunum and ileum by Pili Bacteria synthesize enterotoxins (exotoxins determined by plasmids) Diarrhea • The toxins are strikingly cell-specific: Cells of colon are not susceptible due to lack of receptors for the toxins. • • • • Enteropathogenic E. coli (EPEC) Enterotoxigenic E. coli (ETEC) Enteroinvasive E. coli (EIEC) Enterohemorrhagic E. coli (EHEC) Enteropathogenic E. coli (EPEC) destruction of surface microvilli • fever • diarrhea • vomiting • nausea • non-bloody stools Gut lumen • Diarrhea is self-limited and short duration (1-3 days) 28 Enterotoxigenic E. coli • Travellers diarrhea • Diarrhea like cholera but milder Diarrhea is self-limited and short duration (1-3 days) 29 Enterotoxigenic E. coli (ETEC) • Heat labile toxin (LT) –like choleragen –Adenylate cyclase activated –cyclic AMP concentration –secretion water/ions (potassium and chloride) • Heat stable toxin (ST) – Guanylate cyclase activated – cyclic GMP concentration – uptake water/ions (Sodium and Chloride) 30 Enterohemorrhagic E. coli (EHEC) • Produce verotoxin which works like Shiga toxin • Hemorrhagic – bloody, copious diarrhea – few leukocytes • hemolytic-uremic syndrome – hemolytic anemia – thrombocytopenia (low platelets) – kidney failure Enterohemorrhagic E. coli • Usually O157:H7 Flagella Transmission electron micrograph 32 Enteroinvasive E. coli (EIEC) Very similar to Shigella species (in biochemical and morphological traits) • Non lactose fermantative • Non motile • Invades to epithelial mucosal cells • Cause enteric inflammation E. coli Transmission Meat products or sewagecontaminated vegetables 34 UTI • The most common agent for UTI (Cystitis, pyelonephritis): fever, chills, flank pain • Occurs primarily in women Attributed to 3 features which facilitate ascending infection into bladder: 1. A short urethra 2. Proximity of the urethra to the anus 3. Colonization of vagina by members of fecal flora. • The most frequent cause of nosocomial UTI. (equally in both men and women and associated with using catheters) Systemic infection • Capsule and endotoxin play a prominent role • Capsular polysaccharide interferes with phagocytosis (Serotype having K1 causes neonatal meningitis). • LPS during sepsis causes fever, hypotension and disseminated intravascular coagulation. Treatment • Antibiogram for most infections • A combination of ampicillin and gentamicin in neonatal meningitis • Rehydration for diarrhea Prevention • No passive or active immunisation • Prompt withdraw of catheters and intravenous lines • Caution regarding uncooked food and unpurified water while traveling. Shigella • Important properties - Non lactose fermenting - Distiguishable from Salmonella by: no gas, no H2S, nonmotile. - Divided into 4 groups based on O antigen: A, B, C and D. - Having an enterotoxin called Shiga toxin Disease: Shigellosis • Only a human disease • Transmitted from person to person by asymptomatic carriers (oral fecal transmission) • 4 F’s – fingers, flies, food, feces • Food-born outbreaks outnumber water-born outbreaks by 2 to 1. • In mental hospitals and day-care nurseries • Children <7 accounts for half of shigella positive stool culture Pathogenesis • Exclusively in gastrointestinal tract • Bloody diarrhea (dysentery): Invading the mucosa of the distal ileum and colon. • Local inflammation accompanied by ulceration occurs, but the organisms rarely penetrate the wall or enter the bloodstream unlike salmonellae. • Although some have an enterotoxin, invasion is not only due to enterotoxin. Most invasive species • S. dysenteriae causes the most severe disease but S. sonnei causes mild disease but more frequent Clinical findings • Incubation period: 1-4 days • Symptoms: Fever, abdominal cramps, followed by diarrhea (watery at first but later contains blood and mucus). • It can be mild or severe depending 2 major factor: The species of Shigella and the age of the patient. Clinical findings • Resolves in 2-3 days but antibiotic can shorten the course. • Serum agglutinins appear after recovery but are not protective because the organism does not enter the blood. Lab diagnosis • Non-lactose fermenting…… • Slide agglutination to detect its group • Methylene blue stain of a fecal sample to determine whether PMNs are present: An invasive one: Shigella, Salmonella or Campylobacter rather than a toxin-producing organism such as V. cholerae, E. coli or Clostridium perfringens or certain viruses or Entamoeba histolytica. Treatment • The main treatment: Fluid and electrolyte replacement. • No antibiotic in mild cases • Antibiogram test: Trimethoprim sulfamethoxazole or ampiciln. • Antiperistaltic drugs are contraindicated as they prolong the fever, diarrhea an excretion. Prevention • Interruption of fecal-oral transmission by proper sewage disposal, chlorination of water and personal hygiene. • No vaccine • Antibiotic prophylactic is not recommended. Salmonella Important properties • Not ferment lactose • Produce H2S, Gas, motile Naming the salmonella 1. 2. 3. 4. 5. S. typhi S. paratyphi (A, B, C…) S. typhimurium S. choleraesuis S. enteritidis (1500 serotypes) Diseases - Enterocolitis (S. typhimurium) - Enteric fever (typhoid fever) (S. typhi and S. paratyphi) - Septicemia with metastatic abscesses (S. choleraesuis) Diseases • Enterocolitis An invasion of the epithelial and subepithelial tissue of the small and large intestines. Penetration both through and between the mucosal cells: Inflammation and diarrhea. PMN response limits the infection to the gut and the adjacent mesenteric lymph nodes. The dose of Salmonella required: at least 100,000 while for Shigella: 100 organisms. • Typhoid (Enteric fevers) Infection begins in the small intestine but few gastrointestinal symptoms occur. • The organisms multiply in the mononuclear phagocytes of peyer’s patches, then spread to the phagocytes of the liver, gallbladder and spleen leading to bacteremia and then fever. • Septicemia Accounts for only about 5-10% of Salmonella infections and occurs in: More common in patients with chronic disease or children with enterocolitis. It leads to seeding of many organs, with osteomyelitis, pneumonia, and meningitis as most common sequelac. Clinical findings of Enterocolitis • Incubation period: 6-48 hours Symptoms: Nausea, Vomiting, Abdominal pain and diarrhea with or without blood The disease is self-limited. Treatment only in the very young and very old. S. typhimurium: the most common cause of enterocolitis. Typhoid or Enteric fever • Typhoid or Enteric fever caused by S. typhi and S. paratyphi (A, B and C). - The illness is slow, with fever and constipation rather than vomiting and diarrhea. - After the first week, bacteremia becomes sustained. High fever, tender abdomen, and enlarged spleen occur. Typhoid (continued) • Rose spot (rose-coloured papules) on the abdomen are associated with typhoid fever but occur only rarely. • The disease begins to resolve by the third week but intestinal hemorrage or perforation can occur. • 3% of typhoid fever patients become chronic carriers. The carrier rate is higher among women. Septicemia • S. choleraesuis: most often cause septicemia. • Symptoms: - Fever - Little or no enterocolitis - Focal symptoms: bone, lung, or meninges. Transmission of salmonella • The epidemiology of Salmonella infections: ingestion of food and water contaminated by human and animal wastes. • S. typhi, transmitted only by humans, but other species have a significant animal reservoir. • Human sources are either persons who temporarily excrete the organism during or shortly after enterocolitis or chronic carriers who excrete the organism for years. Transmission • The most frequent animal source is poultry and eggs, but meat products that are inadequately cooked have been implicated as well. • Dogs and other pets including turtles are additional sources. Lab. diagnosis • Enterocolitis: isolated from stool • Enteric fever: blood culture during first 2 weeks of illness. • Septicemia: Blood culture • Lactose -, Gallery, MacConkey • Gas and H2S (S. type: no gas) • Serological tests by their O, H and Vi antigens • Selological detection for Ab if culture is negative. Treatment • Enterocolitis: Self-limited. • Fluid and electrolyte replacement. • Antibiotic can select mutants resistant and increase the frequency of the carrier state. • Antimicrobial agents are indicated only for neonates or persons with chronic disease who are at risk of septicemia and disseminated abscesses. Treatment • Enteric fever and Septicemia: Ampicillin or chloramphenicol. • Ampicillin: in patients who are chronic carriers of S. typhi. • Cholecystectomy may be necessary to abolish the chronic carrier state. • Focal absesses should be drained. Prevention • • • • • • • • Public health and personal hygiene measures. Proper sewage treatment A chlorinated water supply Cultures of stool samples from food handlers Hand washing Pasteurization of milk Proper cooking of poultry and meat Two vaccine confer protection against S. typhi but no common Genus: Yersinia Species: Yersinia pestis and Yersinia enterocolitica • Small gram negative bacillus, bipolar staining (like a safety pin),capsule in freshly isolated organism but lost with passage. Non motile. • Disease Plague or black death Pathogenesis • Urban cycle Transmission of the bacteria among urban rats with the rat flea as vector. This cycle predominates during times of poor sanitation, eg. Wartime, when rats proliferate and come in contact with the fleas in the sylvatic cycle. Epidemiology • Endemic in the wild rodents of Europe and Asia for thousands of years. • 99% of cases of plague occur in Southeast Asia. • Enzootic (sylvatic) cycle consists of transmission among wild rodent by fleas. • Rodents are relatively resistant to disease. • Humans are accidental hosts and cases of plague occur as a result of being bitten by a flea that is a part of the sylvatic cycle. Event within the flea • The flea ingests the bacteria while taking a blood meal from a bacteremic rodent. The blood clots in the flea’s stomach owing to the action of the enzyme coagulase made by the bacteria. The bacteria are trapped in the fibrin and proliferate to large numbers. The mass of organisms and fibrin block the proventriculus of the flea’s intestinal tract. During its next blood meal the flea regurgitates the organisms into the next animal. Because the proventriculus is blocked, the flea gets no nutrition. becomes hungrier loses its natural host selectivity for rodents more readily bites a human. The bacteria inoculated by bite spread to the regional lymph nodes become swollen and tender called buboes and this plague is called bubonic plague. The organisms can reach high concentrations in the blood and disseminate to form abscesses in many organs. The endotoxinrelated symptoms, including disseminated intravascular coagulation and cutaneous hemorrhages, probably were the genesis of the term ‘black death’. Respiratory droplet transmission • Respiratory droplet transmission of the organism from patients with pneumonic plague can occur. Virulence factors • Capsule antigen (F-1) which protects against phagocytosis. • Endotoxin • Exotoxins (block beta adrenergic receptors) • V antigen protein • W antigen protein Clinical findings • Bubonic plague, is the most frequent form, begins with pain and swelling of the lymph nodes draining the site of the flea bite and systemic symptoms such as high fever, myalgias, and prostration. • The buboes are an early characteristic finding. • Septic shock and pneumonia are the main lifethreatening subsequent events. • Pneumonic plague can arise either from inhalation of an aerosol or from septic emboli that reach the lung. • Untreated bubonic plague is fatal in approximately half of the cases. • Untreated pneumonic plague is invariably fatal. Lab. diagnosis • The best procedure: Smear and culture of blood or pus from the bubo. • Giemsa’s or Wayson’s stain reveals the typical safety-pin appearance of the organism better than does Gram’s stain. • Fluorescent-antibody staining can be used to identify the organism in tissues. • A rise in antibody titer to the capsule antigen can be useful. Treatment • The treatment of choice is a combination of streptomycin and tetracycline. • Due to the rapid progression of the disease, treatment should not wait for the results of the bacteriologic culture. • Incision and drainage of the buboes are not usually necessary. Prevention Prevention • Controlling the spread of rats in urban areas. • Preventing rats from entering the country by ship or airplane. • Aviding flea bites. • Avoiding contact with dead wild rodents. • A patient with plague must be placed in strict isolation (quarantine) for 72 hours after antibiotic therapy is started. • Only close contacts need receive prophylactic tetracycline. • There is no vaccine for citizens normally. But a killed organism vaccine protecting bubonic but not pneumonic plague was used by USA forces during Vietnam wars. Vibrionaceae • Curved or “comma-shaped” gram-negative rods. - 2-4 u - Against Enterobacteriaceae, they have monotricus not peritricus flagellum. - All members live in aqueous material. - Genus Vibrio lives in rivers, salt waters like beaches. Vibrio cholerae - Facultative anaerobic Can tolerate pH=9 Fermenting sucrose Oxidase positive Monotricus flagellum Vibrio cholerae • Two groups according to the nature of its O cell wall antigen: - O1 organisms (cause epidemic cholera disease) - Non-O1 organisms (either cause sporadic disease or are non- pathogenes). • O1 organisms: have two biotypes (El Tor and cholerae) and 3 serotypes (Ogawa, Inaba and Hikojima) Epidemiology • Infects only humans • Transmitted by fecal contamination of water and food. • Asymptomatic carriers: individuals in the incubation or convalescing period • Recent pandemic: 1960s-1970s, over 3 continents (Africa, Europe and Asia): El Tor biotype/ usually Ogawa serotype. • Factors predisposing to epidemics: Poor sanitation, malnutrition, overcrowding and inadequate medical services. Quarantine measures failed to prevent the spread of the disease due to many asymptomatic carriers. Pathogenesis • Colonization (approximately 1 billion organism must be ingested, because the organism is particularly sensitive to stomach acid.): Adherence to the cells of brush border of the gut is related to secretion of mucinase: dissolves the glycoprotein coating over the intestinal cells. • Multiplication and secretion of enterotoxin. • Enterotoxin (Choleragen) first binds to a sialic acid-containing ganglioside receptor on the surface of the enterocyte. • Then enterotoxin catalyzes the addition of ADPribose to the coupling protein, resulting in the activation of adenylate cyclase increase in cAMP Secretion of the secretion of H20, Na+, K+, Cl-, and HCO3- into the lumen of the small intestine. • Massive watery diarrhea without inflammatory cells. • Morbidity and death are due to dehydration and electrolyte imbalance. • If treatment is instituted promptly, the disease runs a self-limited course in up to 7 days. Clinical findings • Watery diarrhea in large volumes (the hallmark of cholera) without cells. • Non-bloody rice-water stool • No fever • Symptoms are referable to the dehydration: the loss of fluid and electrolytes leads to cardiac and renal failure. Acidosis and hypokalemia occur as a result of loss of bicarbonate and potassium in the stool. • Mortality rate without treatment is 40% Lab diagnosis • Rice-watery diarrhea with no inflammatory cells. • During an epidemic a clinical judgment is made (little need for the laboratory.) • Colorless colonies on MacConkey’s agar (lactose is fermented slowly). • Culture in selective medium: TCBS (yellow colonies with average size and thin edge) • Oxidase positive (distinguishes it from members of Enterobacteriaceae) • On TSI, Acid/Acid without gas or H2S. • Serologic test (agglutination) • Retrospective diagnosis serologically by detecting a rise in antibody titer in acute and convalescent-phase sera. Treatment • Prompt, adequate replacement of water and electrolytes (orally or intravenously). • Tetracycline is not necessary but do shorten the duration of symptoms and reduce the time of excretion of organisms. Prevention • Providing clean water and food supply. • Vaccine has limited usefulness (50% effective for 3-6 months) • Tetracycline for prevention is effective in close contacts but cannot prevent the spread of a major epidemic. • Prompt detection of carriers is important in limiting outbreaks. Psuedomonaceae • • • • Gran Negative rods Nonfermentative (Strict aerobic) Oxidation of sugars Cytochrome C oxidase Pseudomonas aeroginosa (Piocianic bacillus) • Growth in soil and water containing only traces of nutrients. • A remarkable ability to withstand disinfectants (found in soap solutions, in antiseptics and in detergents). • Persistent in the hospital environment • An important role in hospital-acquired infections P.a. Producing 2 pigments: Pyocyanin (colors the pus in a wound bluegreen) Pyoverdin /Fluorescein (a yellow-green pigment that fluoresces under ultraviolet light In the lab, these pigments diffuse into the agar, imparting a blue-green color that is useful in identification of the species. • In cystic fibrosis patients, P. aeroginosa has a slime layer (glycocalyx): • very mucoidal colonies. The slime layer mediates adherence of the organism to mucous membranes of the respiratory tract and prevents antibody from binding to the organism. Epidemiology • 10% of people carry it in the normal flora of the colon and on the skin in moist areas. • It can colonize the upper respiratory tract of hospitalized patients. • Its ability to grow in simple aqueous solutions has resulted in contamination of respiratory therapy and anesthesia equipments, and even distilled water. • An opportunistic pathogen. e.g. in those with extensive burns (skin host defenses are destroyed), those with chronic respiratory disease (such as cystic fibrosis), immunosuppressed, those with catheters. • 10-20% of hospital-acquired infections. Pathogenesis • Virulance factors: - Endotoxin - Exotoxin A (inhibits eukaryotic protein synthesis by the same mechanism as diphteria exotoxin) Clinical finding • Can cause infections virtually anywhere in the body, but more frequent in: - Urinary tract infections (UTIs) - Pneumonia - External otitis - Wound infections (especially burns). From these sites, the organism can enter the blood, causing sepsis with mortality rate of over 50%. Lab diagnosis • Gram negative rods • Non-lactose-fermenting (colorless) colonies on MacConkey or EMB agar. • In TSI: Alkalin/Alkalin • Oxidase-positive • Blue-green pigment on nutrient agar • Fruity aroma • Catalaze and gelatinase positive Oxidase Test Detecting cytochrome C oxidase enzyme Indicator: 1% tetra methyl-para-phenylene diamine dihydrochloride Treatment • Resistant to many antibiotics • Antibiogram test is essential • Usually is chosen from penicillins or cephalosporins along with an aminoglycoside. Prevention • Keeping neutrophil counts above 500/uL • Removing indwelling catheters promptly • Taking special care of burned skin Spirochetes • • • • • Thin-walled Spiral rods Flexible Motile Having an axial filament under the outer sheath • Treponema - Seen only by darkfield microscopy, silver impregnation, or immunofluorescence. - No growth in bacteriologic media. • Leptospira - Seen only by darkfield microscopy, silver impregnation, or immunofluorescence. - Growth in bacteriologic media • Borreliae - Larger than two others Seen by Giemsa’s and other blood stains Seen in the standard light microscope Growth in bacteriologic media Treponema pallidum pallidum (The bacterial agent of syphilis) • Subspecies pallidum (not bejel, pinta, yaws). • Trasnsmission and Epidemiology - Transmission from spirochete-containing lesions of the skin or mucous membranes - From pregnant women to their fetuses. - Blood transfusion during early syphilis. - A worldwide STD - The incidence is increasing Pathogenesis & Clinical finding • No important toxins or enzymes. • Primary stage Chancre in 2 – 10 weeks (average 21 days). The ulcer heals spontaneously, but spirochetes spread widely in tissues. • Secondary stage: Lesions as maculopapular rash or moist papules on skin and mucous membranes. Or organ involvement (meningitis, nephritis, hepatitis….). • Secondary lesions are rich in spirochetes and highly infectious but heal spontaneously. • Tertiary stage: One-third of early syphilis cases progress to cure without treatment. Another third remain latent; i.e. no lesions appear, but positive serologic tests indicating continuing infections (Asymptomatic). • In the remainder, the disease progresses to the late, tertiary stage. - Granulomas (gummas), especially of skin and bones, central nervous system involvement (e.g. tabes, paresis), or cardiovascular lesions (eg, aortitis, aneurysm). - In tertiary stage, the treponemes are very rare. • - Tertiary stage: Gumma Paralysis Blindness Insanity Death Congenital syphilis • Fetus infection through her/his mother by transplacental passage after the third month of pregnancy. • Unless treated promptly, multiple fetal abnormalities. • Early congenital syphilis occurs in children between 0 and 2 years old. After, they can develop late congenital syphilis. • Symptomatic newborns, if not stillborn, are born premature, with enlargement of the liver, spleen, skeletal abnormalities, pneumonia • malformations of teeth or bones and by active mucocutaneous syphilis at birth or shortly thereafter, and by ocular or neurologic changes. • Babies with congenital syphilis can have symptoms at birth, but symptoms can develop two weeks to three months later, including: • anemia • fever • rashes • skin sores* • swollen liver and spleen • various deformities • weak/hoarse crying sounds • yellowish skin (jaundice) When infected infants become older children and teenagers, late-stage syphilis symptoms may occur, including damage to: bones brain eyes ears Teeth Immunity • Immunity to syphilis is incomplete: Antibodies are produced but not stop the progression of the disease. • Patients with early syphilis who have been treated can contract syphilis again. • Patients with late syphilis are relatively resistant to reinfection. Lab diagnosis • Microscopy Demonstrating spirochetes by darkfield or immunofluorescence microscopy. • Nonspecific serologic tests - Nontreponemal antigens (extracts of normal mammalian tissues. eg. Cardiolipin from beef heart) react with “reagin” antibodies in serum samples from patients with syphilis. - VDRL (Venereal Disease Research Laboratory) - RPR (Rapid plasma reagin) • Antibodies are detectable in the majority of patients at the time the primary lesion appears. • Always present in secondary syphilis. • False-positive reactions occur in infections, such as hepatitis and infectious mononucleosis and in various autoimmune disease. Specific serologic tests • Involving the use of treponemal antigens. • T. pallidum extracted from experimentally infected rabbits reacts in immunfluorescence (FTA-ABS) or hemaglutination (TPHA) tests with specific antitreponemal antibodies, which arise within 2-3 weeks of syphilitic infection. Treatment • Penicillin G (A single injection of benzathin penicillin G) • Tetracycline or erythromycin if given for prolonged periods. Prevention • Administration of antibiotic after suspected exposure. • The presence of any sexually transmitted disease makes testing for syphilis mandatory. • No vaccine is available. Borrelia recurrentis • Causing relapsing fever • During infection, the antigens of these organisms undergo variation. As antibodies develop against one antigen, variants emerge and produce relapses of the illness. • Relapses can be repeated 3-10 times. • Transmission from person to person by human body louse • Humans are the only hosts • Diagnosis - Seeing the large spirochetes in stained smears of peripheral blood. - Culture in special media - Serological tests are rarely useful - Treatment: Tetracycline • Borrelia burgdorferi Transmitted by tick bite Lyme disease • Reservoirs: Mice and deer Rickettsiae • Very short rods and barely visible in the light microscope • Structurally gram-negative but poorly stain with gram stain. • Obligate intracellular parasites, so must be grown in cell culture (The exception is R. quintana). • Against chlamydiae divide by binary division within the host cell not by a distinctive intracellular cycle. Lab diagnosis • The Weil-Felix test detects antirickettsial antibodies in a patient’s serum by agglutination of the proteus organism is based on a cross-reaction, because: Several rickettsiae (R. prowazekii, R. tsutsugamushi, R. ricketsii, …) possess antigens that cross-react with antigenes of OX strains of proteus vulgaris. Transmission • They maintained in nature in certain arthropods, such as ticks, lice, fleas and mites and with one exception are transmitted to humans by the bite of the arthropod. • The exception to arthropod transmission is C. burnetii, the cause of Q fever, which is transmitted by aerosol and inhaled into the lungs. • Virtually all rickettsial diseases are zoonoses with the exception of epidemic typhus which occurs only in humans. • R. prowazekii Epidemic Typhus (by lice) • R. typhi Typhus (by lice) • R. tsutsugamushi Typhus (by lice) • Rickettsia rickettsii Rocky mountain spotted fever (by ticks) • Coxiella burnetii Q fever (inhalation, contact with the milk, urine, feces, vaginal mucus, or semen of infected animals.