* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 8.1 Metabolism
Survey
Document related concepts
Transcript
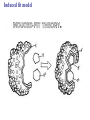
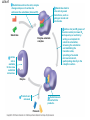
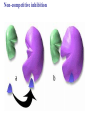
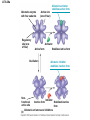
Essential Idea • Metabolic reactions are regulated in response to the cell’s needs. Understandingis • Metabolic pathways consist of chains and cycles of enzyme-catalysed reactions. • Enzymes lower the activation energy of the chemical reactions that they catalyse. • Enzyme inhibitors can be competitive or noncompetitive. • Metabolic pathways can be controlled by endproduct inhibition. Skills & Application • Application: End-product inhibition of the pathway that converts threonine to isoleucine. • Application: Use of databases to identify potential new anti-malarial drugs. • Skill: Calculating and plotting rates of reaction from raw experimental results. • Skill: Distinguishing different types of inhibition from graphs at specified substrate concentration. Guidance • Enzyme inhibition should be studied using one specific example for competitive and noncompetitive inhibition. IB Assessment Statement • State that metabolic pathways consist of chains and cycles of enzyme catalyzed reactions. State that metabolic pathways consist of chains and cycles of enzyme catalyzed reactions. 7.6.1 Metabolic pathways. • Chemical changes in living things often occurring with a number of intermediate stages. • Each stage has its own enzyme. • Catabolic pathways breakdown molecules • Anabolic pathways build up molecules State that metabolic pathways consist of chains and cycles of enzyme catalyzed reactions. 7.6.1 Metabolic pathways Animation --Click here for Animation: http://highered.mcgrawhill.com/sites/0072507470/student_view0/chapter2/animation __a_biochemical_pathway.html State that metabolic pathways consist of chains and cycles of enzyme catalyzed reactions. Two Types Metabolic pathways. • Linear Pathway • Cyclical pathway Linear metabolic pathways. • Enzyme (1) is specific to substrate 1. This is changed to product 1. • Enzyme (2) is specific to product1 which becomes the substrate and converted to product 2. • Enzyme (3) is specific to products which becomes the substrate and converted t o product 3. • Product 3 is called the 'End product'. Cyclic metabolic pathways. • The initial substrate is fed into the cycle. • Enzyme (1) combines the regenerated 'intermediate 4' with the initial substrate to catalyses the production of intermediate 1. • Enzyme (2) is specific to intermediate 1 and converts intermediate 1 to intermediate 2 • Enzyme (3) is specific to intermediate 2 and catalyses it conversion to product and intermediate 3. • Enzyme (4) is specific to intermediate 3 and catalyses its conversion to intermediate 4. • The difference is the regeneration of the intermediate, in this case intermediate 4. IB Assessment statement Describe the induced-fit model. • This is an extension of the lock-and-key model. • Its importance in accounting for the ability of some enzymes to bind to several substrates should be mentioned. Induced fit model • Induced fit model - A model for enzymesubstrate interaction • states only the correct substrate is capable of inducing the correct shape of the active site • This change in shape allows the enzyme to perform its catalytic function. • Induced Fit Animation Click here: http://www.stolaf.edu/people/giannini/flashanimat/enzymes/enzyme.swf Induced fit model LE 8-17 Substrates enter active site; enzyme changes shape so its active site embraces the substrates (induced fit). Substrates held in active site by weak interactions, such as hydrogen bonds and ionic bonds. Substrates Enzyme-substrate complex Active site is available for two new substrate molecules. Enzyme Products are released. Substrates are converted into products. Products Active site (and R groups of its amino acids) can lower EA and speed up a reaction by • acting as a template for substrate orientation, • stressing the substrates and stabilizing the transition state, • providing a favorable microenvironment, • participating directly in the catalytic reaction. IB Assessment Statement Explain that enzymes lower the activation energy of the chemical reactions that they catalyse. – Only exothermic reactions should be considered. Specific energy values do not need to be recalled. Exergonic and Endergonic Reactions in Metabolism • An exergonic reaction proceeds with a net release of energy • An endergonic reaction absorbs energy from its surroundings Exothermic Reaction Free energy Reactants Amount of energy released (G < 0) Energy Products Progress of the reaction Exergonic reaction: energy released LE 8-6b Endogonic Reaction Free energy Products Energy Reactants Progress of the reaction Endergonic reaction: energy required Amount of energy required (G > 0) Effect of Catalyst on Activation Energy Enzymes lower the activation energy of the chemical reaction that they catalyze. • In the activated complex or transition state energy is put into the substrate to weaken the structure. This allow the reaction to occur with a minimal amount of additional energy required. • Normal activation energy would cause damage to the proteins of the cell. Thus reduced activation energy make these reactions possible in a cell. • After the product is formed energy is released. • Exergonic reactions release more energy than the activation energy. . LE 8-14 A B C D Free energy Transition state A B C D EA Reactants A B G < O C D Products Progress of the reaction LE 8-15 Free energy Course of reaction without enzyme EA without enzyme EA with enzyme is lower Reactants Course of reaction with enzyme G is unaffected by enzyme Products Progress of the reaction IB Assessment Statement • Explain the difference between competitive and non-competitive inhibition, with reference to one example of each. Enzyme Inhibitors • Competitive inhibitors bind to the active site of an enzyme, competing with the substrate • Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective The inhibitor has a similar shape to the usual substrate for the enzyme, and competes with it for the active site The activity of the enzyme is inhibited till the inhibitor has a dissociated. Non-competitive inhibition • Substrates and inhibitors do not have a similar structure. • Inhibitors bind at a different site from the active site • Inhibitors hence change the conformation of the enzyme. • Substrate may/may not bind • Active site doesn’t catalyze the reaction Non-competitive inhibition Non-competitive inhibition A substrate can bind normally to the active site of an enzyme. Substrate Active site Enzyme Normal binding A competitive inhibitor mimics the substrate, competing for the active site. Competitive inhibitor Competitive inhibition A noncompetitive inhibitor binds to the enzyme away from the active site, altering the conformation of the enzyme so that its active site no longer functions. Noncompetitive inhibitor Noncompetitive inhibition Allosteric Regulation of Enzymes • Allosteric regulation is the term used to describe cases where a enzyme’s function at one site is affected by binding of a regulatory molecule at another site • Allosteric regulation may either inhibit or stimulate an enzyme’s activity Allosteric Activation and Inhibition • Each enzyme has active and inactive forms • The binding of an activator stabilizes the active form of the enzyme • The binding of an inhibitor stabilizes the inactive form of the enzyme LE 8-20a Allosteric enzyme with four subunits Regulatory site (one of four) Active site (one of four) Activator Active form Oscillation Nonfunctional active site Allosteric activator stabilizes active form. Inactive form Stabilized active form Allosteric inhibitor stabilizes inactive form. Inhibitor Allosteric activators and inhibitors Stabilized inactive form LE 8-20b Binding of one substrate molecule to active site of one subunit locks all subunits in active conformation. Substrate Inactive form Stabilized active form Cooperativity another type of allosteric activation IB Assessment Statement • Explain the control of metabolic pathways by end-product inhibition, including the role of allosteric sites Feedback Inhibition • In feedback inhibition, the end product of a metabolic pathway shuts down the pathway • Feedback inhibition prevents a cell from wasting chemical resources by synthesizing more product than is needed • Click here for Animation: http://highered.mcgrawhill.com/sites/0072507470/student_view0/chapter2/animation__fee dback_inhibition_of_biochemical_pathways.html Feedback Inhibition Initial substrate (threonine) Active site available Isoleucine used up by cell Threonine in active site Enzyme 1 (threonine deaminase) Intermediate A Feedback Enzyme 2 inhibition Active site of enzyme 1 can’t bind Intermediate B theonine pathway off Enzyme 3 Isoleucine binds to allosteric site Intermediate C Enzyme 4 Intermediate D Enzyme 5 End product (isoleucine) Feedback Inhibition a detailed example 1. Isoleucine the end product, this molecule can inhibit the enzyme Threonine Deaminase 2. The inhibition occurs at an inhibition site on the enzyme but not the active site 3. An excess of end product (Isoleucine) switches off any more production of that product, isoleucine. 4. At high concentrations, Isoleucine attaches to the inhibition site of Threonine deaminase. 5. This attachment causes the active site of the enzyme to change blocking any further reaction. 6. Isoleucine is used up in cellular processes that require this particular amino acid. Feedback Inhibition a detailed example 7. The isoleucine concentration in the cell falls and so the Isoleucine that is attached to the enzyme detaches. This amino acid is also used up in the various cellular processes. 8. With the inhibitor removed the the active site then becomes active again and the pathway switches back on. 9. The isoleucine is again in production but once high concentrations are reached the pathways is once more inhibited. The process then cycles on in alternating stages of production and inhibition 10. Notice the similarity with non-competitive inhibition. 11. This mechanism makes the pathway self-regulating in terms of product manufacture.