* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Survey
Document related concepts
Transcript
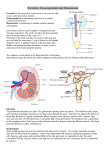
Urinary System L 4 Acid Base Balance and Avian Urinary System Prof. Madaya Dr Than Kyaw 15 October 2012 More about renal clearance Creatinine clearance: Creatinine: a nitrogenous by-product of muscle metabolism - naturally found in the blood - constantly produced - constantly excreted - no need to infuse exogenous creatinine for testing - more frequently used as clinical renal function test in animals - normal plasma level = 0.5 to 2.0 mg/dL - not accurate as inulin (about 10% of creattinine is reabsorbed) More about renal clearance Creatinine clearance: - it is used for GRF because of direct relationship with the functional renal mass - loss of nephron numbers by kidney disease can be confirmed by decrease in GFR - normal endogenous creatinine GFR in dog = between 2 and 4 ml/min/kgBW More about renal clearance Creatinine clearance: Determination 1. 2. 3. 4. 5. Collection of urine for a 24 h period Vol collected is divided by 1440 to get urine flow rate (V) in ml/min Determination of creatinine conc for urine (U) and plasma (P) The product of urine conc (U) and volume (V) provides excretion rate The quotient obtained from UV/P is further divided by body weight in kg to give GFR in mg/min/kg BW 6. Increasing value for P results in decreasing value in C (GFR) More about renal clearance Creatinine clearance: Example Data in a healthy 14 kg dog V = Urine flow rate = 280 ml/1440 min = 0.194 ml/min U = Urine creatinine conc = 150 mg/dL =1.5 mg/ml P = Plasma creatinine conc = 0.6 mg/dL = 0.006 mg/ml Calculation U x V = creatinine excreation rate = 1.5 mg/ml 0.194 ml/min = 0.291 mg/min C = UV/P = (0.291 mg/min)/0.006 mg/ml = 48.5 ml/min GFR = C/kgBW = (48.5 ml/min)/14 kg = 3.46 ml/min/kg Normal values for endogenous creatinine clearance in dog 2.98 0.96 ml/min/kg Acid-base balance in the body - Body fluid - relatively constant H+ concentration - It is the result of a balance between acids and bases. - Disturbance of the balance - when acids or bases added to or removed from the body fluids Acidemia – depression of blood pH below normal Alkalemia – a value above normal pH Acidosis – addition of excess acids or removal of base from ECF Alkalosis – addition of excess base or loss of acid Acid-base balance in the body - Under normal condition – acids or bases are continuously added to the body fluid due to cellular metabolism or ingestion Diseases causing – respiratory insufficiency - renal insufficiency - vomiting - diarrhoea Loss or gain of acids or bases Acid-base balance in the body Normal ECF value of pH = 7.4 (narrow range 7.35 – 7.45) pH value of ECF very sensitive A pH change of 0.3 units doubles or halves the H+ concentration pH 7.4 7.7 7.1 H- concentration 40 ngEq/L 80 nEq/L 20 nEq/L Condition Normal pH Severe alkalosis Severe acidosis Acid-base balance in the body Three basic mechanisms involved in acid-base balance 1. Chemical buffering 2. Respiratory adjustment of blood CO2 concentration 3. Excretion of H+ or HCO3- by the kidney Na+ and H + countertransport Renal H+ Secretion mechanism - Epithelial cells throughout the length of the nephron (except thin segment of loop of Henle) – secretes H+ - 85% secreted by proximal tubules - Hydration reaction ← ← → + → - Reaction in cytoplasm of tubular epithelial cells - Need enzyme – carbonic anhydrase (brush border) - CO2 – freely diffuse from ECF into cells - After hydration – H+ formed secreted into the lumen in exchange for a Na+ (countertransport) - H+ combines with bicarbonate tubular buffer forming – H2CO3 - H2CO3 further dehydrated to CO2 and H2O – part of urine + Renal H+ Secretion mechanism - HCO3 – formed within the cell difuses into ECF accompanied by Na+ exchanged for H+ tubular epithelial cells - ECF loses a H+ and gains a HCO3 – - Gain of HCO3 – (into ECF)and loss of HCO3 – (from tubular fluid) balance each other – maintain pH equilibrium - In excess H+ production – phosphate buffer is used for exchange with H+ - If acidosis persists – formation and secretion of ammonia by tubular epithelial cells increases – letting H+ to be secreted continuously without lowering pH of tubular fluid Buffering with phosphate Buffering with ammonia Respiratory system in acid-base balance - Equally important for the maintenance of acid-base - During transport from body cells to lungs, CO2 diffuses into RBCs - Hydration reaction – forming H+ and HCO3 – - H+ is buffered and HCO3 – diffuses into the plasma - In lung CO2 diffuses into alveoli and reverse hydration equation - losing H+ from ECF CO2 in RBC=93% Lung CO2 released in tissues are carried to lung in 3 forms: (1) Dissolved form in plasma (2) Bound to Hb (3) As bicarbonate in plasma ( in the lung, reaction is reversed to release CO2) Chemical buffering system - The first line of defense in maintaining constant pH of ECF - Includes - bicarbonate, phosphate and proteins Bicarbonate System HCl + NaHCO3 = H2CO3 + NaCl - formation of a weaker acid and salt NaOH + H2CO3 = NaHCO3 + H2O - Weak acid reacts with a base to form a weaker base and water Chemical buffering system Phosphate buffer system Similar action to acid and base HCl + Na2HPO4 = NaH2PO4 + NaCl NaOH + NaH2PO4 = Na2HPO4 + H2O - Proteins react as buffers. - it has large number of acidic and basic groups. - The basic group (R-NH2) acts as buffer by taking up H+ forming (R-NH3- ) - Acidic groups (R-COOH) act as buffers by losing H+ and forming an ion (RCOO –) Relative merits of Buffer systems Bicarbonate buffering system: - weak - but unique as it involves both in respiratory and kidneys - components are elements of hydration reaction. Concentration of phosphate buffer - relatively low in ECF - higher in intracellular fluid - important for intracellular buffer - also important for buffering renal tubular fluids when H+ is secreted Protein buffers - abundance in body cells, plasma and Hb), - anemic animals - (low Hb) – quickly become acidic when is secreted - Hb the most abundant chemical buffer in the body Buffer systems do not act separately, they buffer at the same time. The buffers buffer the buffers. Avian Urinary System Avian Urinary System • Many similarities and dissimilarities bet: birds and mammals Similarities Urine formation • Glomerular filtration • Tubular reabsorption • Tubular secretion Also – Birds can modify ureteral urine so that it may have an osmolality that is above or below plasma. Avian Urinary System • Many similarities and dissimilarities bet: birds and mammals Dissimilarities from mammals Birds • Presence of 2 major nephron types • Presence of renal portal system • Formation of uric acid instead of urea as major end product of nitrogen metabolism • Post-renal modification of ureteral urine Avian Urinary System: functional anatomy • Paired as in mammals • Each kidney: 3 lobes – cranial, middle, caudal • Ureters transport urine from the kidneys to cloaca (not present in mammal; no bladder in birds) • Common opening for many ducts – what are they? • Lobes -- lobules Avian Urinary System: functional anatomy • A lobule – appearance of a mushroom with cortex corresponding to the cap of mushroom and the medulla corresponding to the stem Two types of nephron Reptilian type nephron (RTN) - lack loops of Henle - located in the cortex - incapable of concentrating urine Mammalian type nephron (MTN) - well-defined loops of Henle - grouped into a medullary cone, the part of lobule correspond to the stem of a mushroom - collecting ducts and vasa recta also in the cone a. Reptilian type nephron: simple looping pattern and lack of cross-branching in these capillaries b. Mammalian type nephron: with a longer, more complex capillary network. Avian Urinary System: functional anatomy • Avian kideny – capable of alternating the use of reptilian or mammalian type nephrons depending on need of water conservation • MTN use - for greater water conservation • When both types are functional: - 25% of filtrate by MTN - 75% of filtrate by RTN Avian Urinary System: functional anatomy Renal portal system - Unique feature of avian kidey - It is venous blood coming to the kidney from the hind limbs via the external iliac and sciatic veins - Supplies afferent blood to the peritubular capillaries and to efferent arteriolar blood and finally to central vein of the lobule - Renal portal system supplies - ½ to 2/3 of renal blood - Renal portal valves – located at the junction of right and left renal veins – more blood to renal portal system Renal portal system Intralobular blood flow in the kidney of birds Uric acid formation and excretion - Protein and amino acid metabolism - nitrogenous end products - Two-third or more of them – excreted as urea or uric acids - Urea and uric acid less toxic than ammonia - In reptiles and birds – uric acid is formed instead of urea - urea has osmotic effect than uric acid Uric acid formation and excretion - uric acid, when in excess, - is precipitated which has no effect of osmotic pressure – therefore - no water loss for its excretion - It is important for water conservation. - More important during embryonic development inside the egg - If urea is formed -necessary to eliminate liquid urine – not possible within the egg - Uric acid is excreted as white coagulum. - Uric acid is formed in the liver and kidney in birds