* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemistry 1- Separation
Survey
Document related concepts
Transcript
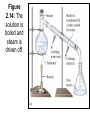
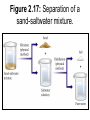
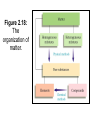
Chemistry 1- Separation Objectives: 1) Learn about 2 different separation methods Two methods to separate mixtures • Distillation: The process by which a mixture is separated by heating a solution and condensing using a cooling tube. Figure 2.14: The solution is boiled and steam is driven off. Figure 2.14: Salt remains after all water is boiled off. Two methods to separate mixtures • Filtration: Pour the mixture through a mesh/or filter paper which allows the liquid to pass through and leaves the solid behind Figure 2.16: Filtration separates a liquid from a solid. Quiz • You have the following mixture. – Gravel, sand and salt – Describe in steps how you would separate this mixture. – Be sure to name the appropriate separation technique. Other Separation Techniques • Chromatography – Paper chromatography (chlorophyll, pigments – Thin Layer chromatography (lipids) – HPLC (high pressure liquid chromatography) – Gas chromatography • Mobile phase/Stationary Phase – Mobile phase: isopropyl alcohol – Stationary phase: T-shirt Uses for Chromatography Type of Chromat ography Applications in the Real World Why and What is it Liquid Chromat ography test water samples to look for pollution, Used to analyze metal ions and organic compounds in solutions. It uses liquids which may incorporate hydrophilic, insoluble molecules. Gas Chromat ography detect bombs in airports, identify and quantify such drugs as alcohol, used in forensics to compare fibers found on a victim Used to analyze volatile gases. Helium is used to move the gaseous mixture through a column of absorbent material. Thin-Layer Chromatograp hy detecting pesticide or insecticide residues in food, also used in forensics to analyze the dye composition of fibers Uses an absorbent material on flat glass plates. This is a simple and rapid method to check the purity of the organic compound. separating amino acids and anions, RNA fingerprinting, separating and testing histamines, antibiotics The most common type of chromatography. The paper is the stationary phase. This uses capillary action to pull the solutes up through the paper and separate the solutes. Paper Chromat ography Types of Chromatography • • • • • • • • • • Adsorption Chromatography Adsorption chromatography is probably one of the oldest types of chromatography around. It utilizes a mobile liquid or gaseous phase that is adsorbed onto the surface of a stationary solid phase. The equilibriation between the mobile and stationary phase accounts for the separation of different solutes. Partition Chromatography This form of chromatography is based on a thin film formed on the surface of a solid support by a liquid stationary phase. Solute equilibriates between the mobile phase and the stationary liquid. Ion Exchange Chromatography In this type of chromatography, the use of a resin (the stationary solid phase) is used to covalently attach anions or cations onto it. Solute ions of the opposite charge in the mobile liquid phase are attracted to the resin by electrostatic forces. Molecular Exclusion Chromatography Also known as gel permeation or gel filtration, this type of chromatography lacks an attractive interaction between the stationary phase and solute. The liquid or gaseous phase passes through a porous gel which separates the molecules according to its size. The pores are normally small and exclude the larger solute molecules, but allows smaller molecules to enter the gel, causing them to flow through a larger volume. This causes the larger molecules to pass through the column at a faster rate than the smaller ones. Affinity Chromatography This is the most selective type of chromatography employed. It utilizes the specific interaction between one kind of solute molecule and a second molecule that is immobilized on a stationary phase. For example, the immobilized molecule may be an antibody to some specific protein. When solute containing a mixture of proteins are passed by this molecule, only the specific protein is reacted to this antibody, binding it to the stationary phase. This protein is later extracted by changing the ionic strength or pH. Figure 2.17: Separation of a sand-saltwater mixture. Figure 2.18: The organization of matter.