* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Nucleation Rates of Supercooled Water
Sessile drop technique wikipedia , lookup
Liquid crystal wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Transition state theory wikipedia , lookup
Ultrahydrophobicity wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
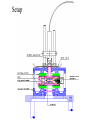
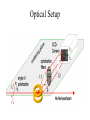
Freie Universitaet Berlin Department of physics and phys. chemistry. The Homogeneous Nucleation Rates of Supercooled Aqueous Non-Electrolytic Mixtures Petr Kabath SET for Europe August 2005 Brno Why nucleation? It is important in the processes in the atmosphere like creation of clouds. The nucleation is of a great importance for the species living under extremal conditions like polar fishes (Arctic Cod is on the figure), insects or plants. It must be prevented or inhibited. Outline • How does it freez? Brief description of nucleation • Electrodynamical trap and scatterd light • The rates of homogeneous nucleation (results) • Summary and outlook How does it freez? • We investigate homogeneous nucleation rates. • The solid phase embryos are created spontaneous in the liquid phase. • If the embryo reaches a critical size then it continues to grow. If the critical size is not reached then it shrinks. G is an energy of formation of a critical germ Freezing of water Creation of the critical nucleus leading to freezing. of water with 512 molecules. Computation by Matsumoto, Saito and Ohmine, Nature, Vol. 416, 2002 Nucleation statistics • Freezing process in a bulk volume is a stochastic process and it is described by the Poisson distribution dN JV f N dt J is the rate constant of the freezing process What do we need for evaluating of J? • The nucleation rate J could be estimated from the equation N ln u JVt N0 where V is the freezing volume and t is the nucleation time, N0 is the number of all droplets and Nu is the number of unfrozen droplets. • Thus we need to estimate V and t from the experiment. Electrodynamical trap and scattered light (Experimental part) • Electrodynamical trap • Optical setup and Mie-Scattering • Measurement Naked Trap The „hearth“ of an experimental setup is the inner chamber with ring electrodes. Potential in the trap Voltage in the trap has a form of U U0 cos t U DC This gives us a time dependent field with a zero potential in the middle of the trap. The droplet moves on the Lisajous curve but for our purposes it is stable. Setup Creation and trapping of the droplet • The quality of the droplet is controled by stroboscope effect. The droplet injection frequency is synchronized with the frequency of the AC voltage in the trap. • Usual droplet diameter varies between 120-30 micrometers Mie Scattering • if the drop is liquid then the laser light has the same polarization as before the interaction with the drop • if the droplet freezes then the laser light will become depolarized • number of Mie stripes is proportional to the diameter of the drop Optical Setup Phase transition The rates of homogeneous nucleation (Data processing) Nucleation rate J is the slope of the logarithmic equation for Nu/N0 Factors influencing the measurements • The diameters of the generated droplets are not constant • Evaporation The rates of homogeneous nucleation for water Homogeneous nucleation rates A concentration dependency of the mixture on the supercooling for a given value of J. • the distribution of the clusters of the mixture molecules probably do not influence the linearity or they are statisticaly homogeneous mixed The rates of homogeneous nucleation a comparison The most recent measurements • Measurements of rates of homogeneous nucleation of simple alcohol-water mixtures • Measurements of dioxane-water mixtures Summary and outlook • precise method for measurements of homogeneous nucleation rates • non-contact method • we have two traps currently, one fully automatised • measurements of alcohol-water mixtures, dioxane-water mixtures, some biological relevant mixtures (amino acids) Acknowledgements • To Prof. H. Baumgaertel • To my collegues I. R. Türkmen, A. Lindinger who are involved within this project • Especially to P. Stöckel for his help with the operating of the trap • To Mr. E. Biller for the technical support and for all perfectly running injectors • To DFG for the financial support Our publications 1. 2. 3. 4. 5. 6. B. Krämer, O. Hübner, H. Vortisch, L. Wöste, T. Leisner, M. Schwell, E. Rühl, H. Baumgärtel: Homogeneous Nucleation Rates of Supercooled Water Measured in Single Levitated Microdroplets, J. Chem. Phys, 111, (1999), 6521 H. Vortisch, B. Krämer, I. Weidinger, L. Wöste, T. Leisner, M. Schwell, H. Baumgärtel, E. Rühl: Homogeneous freezing nucleation rates and crystallization dynamics of levitated sulfuric acid droplet PCCP 7, (2000), 1407 P. Stöckel, H. Vortisch, T. Leisner, H. Baumgärtel: Homogeneous Nucleation of Supercooled Liquid Water in Levitated Microdroplets, J. Mol. Liq., 96-97, (2002), 153 I. Weidinger, J. Klein, P. Stöckel, and H. Baumgärtel, T. Leisner: Nucleation Behavior of n-Alkane Microdroplets in an Electrodynamic Balance, J. Phys. Chem. B, 107, (2003), 3636 Stöckel P., Inez M. Weidinger, Helmut Baumgärtel, Thomas Leisner: Rates of homogeneous ice nucleation in levitated H2O and D2O Droplets, J. Phys. Chem. A, 109 (2005), 2540 P. Stoeckel, P. Kabath, A. Lindinger and H. Baumgaertel: A kinetic two-step model of homogeneous nucleation of ice in supercooled supercooled liquid H2O and D2O, paper submitted to J. Mol. Liq. The device itself