* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lithium Adverse Effects
Pharmacognosy wikipedia , lookup
Psychopharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug design wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropharmacology wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Drug interaction wikipedia , lookup
Lithium (medication) wikipedia , lookup
Dydrogesterone wikipedia , lookup
Pharmacokinetics wikipedia , lookup
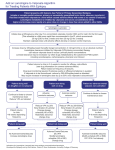
Pharmacologic Considerations in the Treatment of Bipolar Affective Disorder Presented by: Ann M. Hamer, PharmD, BCPP Date: 11/6/2014 Disclosures and Learning Objectives • Learning Objectives – – – Be able to recognize common adverse effects of mood stabilizers Be able to identify common drug interactions associated with mood stabilizers Be able to discuss differences between the available mood stabilizers Disclosures: Dr. Ann Hamer has nothing to disclose. Pharmacologic Treatment of Bipolar Disorder • • • • • Review adverse effects Review drug interactions Review dosing Review monitoring parameters Review comparative cost Treatment Recommendations Acute Mania Stop antidepressants (or inciting agents) Use a mood stabilizer first: Lithium, Valproate Carbamazepine, Oxcarbazepine If psychosis occurs, use an antipsychotic: Olanzapine, Risperidone, Asenapine Aripiprazole, Ziprasidone, Quetiapine Consider short term use of a benzo Acute Depression Start with lithium or lamotrigine (alternatives include: quetiapine, olanzapine/fluoxetine) “Antidepressant monotherapy is not recommended.” Add lamotrigine or bupropion if needed ECT if severely depressed or pregnant CBT and Behavioral Activation Rapid Cycling Identify and treat comorbid contributors such as hypothyroidism or drug/alcohol use Taper contributing medications Valproate often preferred (alternatives include carbamazepine, lithium, or lamotrigine) Combination treatment often required Maintenance Continue agent that helped in acute phase Taper benzodiazepines Taper antipsychotics when mood stable Lamotrigine and lithium may help ward off depression Lithium may be better at warding off mania Valproate, Olanzapine, Carbamazepine, Oxcarbazepine also evidence-based Lithium Lithium Adverse Effects • Common: Leukocytosis (most patients), Polyuria/polydipsia (3050%), Dry mouth (20-50%), Hand tremor (45% initially, 10% after 1 year of treatment), Confusion (40%), Decreased memory (40%), Headache (40%), Muscle weakness (30% initially, 1% after 1 year of treatment), Electrocardiographic (ECG) changes (20-30%), Nausea, vomiting, diarrhea (10-30% initially, 1-10% after 1-2 years of treatment), Hyperreflexia (15%), Muscle twitch (15%), Vertigo (15%) • Less common: Extrapyramidal symptoms, goiter (5%), Hypothyroidism (1-4%), Acne (1%), Hair thinning (1%) • Black Box Warning: Toxicity with higher plasma concentrations • Pregnancy Category D Lithium Drug Interactions • Caution with medications known to prolong the QT interval • Caution with other medications that may increase serotonin levels • Caution with medications/conditions that affect renal clearance (e.g. thiazides, pregnancy) Lithium Dosing • Immediate release: 900-2400 mg/day PO divided q6-8hr • Extended release: 900-1800 mg/day PO divided q12hr • Lower initial dosage may be used to minimize adverse drug reactions Lithium Monitoring • • Lithium levels • T1/2 = 18-24 hours; ss 4-5 days; draw levels 12 hours post-dose, usually prior to the morning dose • Target level = 0.6 -1.2 mEq/L; should not exceed 1.5mEq/L • LiCp < 1.5 mEq/L (mild toxicity and transient effects) = Fine hand tremor, GI upset (N/V/D/anorexia), mild polyuria, polydipsia, muscle weakness • LiCp = 1.5 – 2.5 mEq/L (moderate toxicity) = Course hand tremor, twitching, reoccurrence of GI upset, slurred speech, vertigo, confusion, sedation, lethargy, hyperreflexia • LiCp > 2.5 mEq/L (severe toxicity) = Seizures, stupor, coma, cardiovascular collapse, death Other common monitoring parameters: pregnancy test at baseline; Ca, Cr, urinalysis, TSH at baseline, then at least q6-12mo; serum drug levels 2x/wk until stable, then q2mo until chronic steady dose, then q6-12mo; ECG at baseline in pts >40 yo or if cardiovascular dz, then q6-12mo; consider CBC at baseline Valproate Valproate Adverse Effects • Common : Headache (31%), Asthenia (27%), Somnolence (27%), Tremor (25%), Dizziness (25%), Diplopia (16%), Amblyopia/blurred vision (12%), Nausea (48%), Vomiting (27%), Abdominal pain (23%), Diarrhea (13%), Anorexia (12%), Flu syndrome (12%), Infection (12%) • Less Common: Dyspepsia (8%), Ataxia (8%), Nystagmus (8%), Fever (6%), Emotional lability (6%), Thinking abnormal (6%), Alopecia (6%), Weight loss (6%), Constipation (5%), Amnesia (5%), Bronchitis (5%), Rhinitis (5%) • Black Box Warnings: Hepatotoxicity, teratogenicity, pancreatitis • Pregnancy Category: D Valproate Drug Interactions • CYP 450: CYP2C9 inhibitor, weak; 3A3/4 inhibitor, weak • UGT2B7 inhibitor • antiplatelet effects • CNS depression • hyperammonemia • hyponatremia Valproate Dosing • Depakote initial dose: 750 mg/day PO in divided doses • Depakote ER initial dose: 25 mg/kg PO once daily. Increase as rapidly as possible to achieve the lowest therapeutic dose that provides desired clinical effect or plasma concentration • Doses should not exceed 60 mg/kg/day Valproate Monitoring • Valproate Levels • Target plasma levels = 50 - 125 or 150 mcg/mL • Draw level prior to giving a dose • SS within 1-4 days; draw level after 3rd or 4th day • Other common monitoring parameters: LFTs at baseline, then frequently, especially during 1st 6mo or if suspected hereditary mitochondrial dz; Plt, coagulation tests at baseline, then periodically, also before planned surgery; serum drug levels; ammonia; s/sx depression, behavior changes, suicidality Lamotrigine Lamotrigine Adverse Effects • Common : Dizziness (38%), Diplopia (26-30%), Headache (29%), Ataxia (22%), Blurred vision (16-20%), Rhinitis (11-15%), Somnolence (14%) • Less Common: Insomnia (6-10%), Fatigue (8%), Chest pain (5%), Peripheral edema (2-5%), Suicidal ideation (2-5%), Dermatitis (25%), Dry skin (2-5%), Increased libido (2-5%), Rectal hemorrhage (2-5%), Weakness (2-5%), Agitation (1-5%), Dysarthria (1-5%), Edema (1-5%), Fever (1-5%), Migraine (1-5%), Abnormal thoughts (1-5%), Urinary frequency (1-5%), Tremor (4%) • Black Box Warnings: Serious rash (Stevens-Johnson syndrome) • Pregnancy Category: C Lamotrigine Drug Interactions • Not a CYP inhibitor or inducer, but metabolism is affected by inhibitors and inducers. • Caution when used in combination with valproate/valproic acid • UGT1A4 substrate Lamotrigine Dosing • Monotherapy or without enzyme inducers or valproic acid • Initial: 25 mg PO qDay for 2 weeks, then 50 mg PO qDay for 2 weeks; 100 mg PO qDay for 1 week; Double dose qWeek to maintenance at 200 mg/day PO • With AED regimen without valproic acid • Initial: 50 mg PO qDay for 2 weeks, then 100 mg/day PO divided q12hr for 2 weeks; Increase by 100 mg qWeek to 400 mg/day PO divided q12hr • With valproic acid • Initial: 25 mg PO qOD for 2 weeks, then 25 mg PO qDay for 2 weeks; Double dose qWeek to maintenance at 100 mg/day PO Lamotrigine Monitoring • No specific plasma concentration monitoring • Other common monitoring parameters: Cr at baseline; ophthal. exams if prolonged tx; s/sx depression, suicidality, clinical worsening if bipolar disorder, and/or unusual behavior changes Carbamazepine Carbamazepine Adverse Effects • Common : Ataxia (15%), Dizziness (44%), Drowsiness (32%), Nausea (29%), Vomiting (18%) • Less Common: Dry mouth (8%) • Rare: MI, Stevens-Johnson syndrome, Hepatic failure, Punctate cortical lens opacities, Syndrome of inappropriate antidiuretic hormone secretion (SIADH) • Black Box Warnings: Serious and sometimes fatal dermatologic reactions; aplastic anemia, agranulocytosis • Pregnancy Category: D Carbamazepine Drug Interactions • CYP 450 • CYP2C8 substrate, CYP3A4 substrate; CYP1A2/2A6 inducer, minor; CYP2B6 inducer, minor;CYP2C8 inducer, minor; CYP2C9 inducer, minor; CYP3A4 inducer, major; UGT1A4 inducer;P-gp (MDR1) inducer; • CNS depression • GI absorption enhanced by GLP-2 receptor agonist • hyperammonemia • hyponatremia • increases thyroid hormone clearance • serotonergic effects, weak Carbamazepine Dosing • Initial: 200 mg PO q12hr • Increase by increments of 200 mg/day; not to exceed 1600 mg/day Carbamazepine Monitoring • Target plasma concentration is 8-12 mcg/mL • Toxic Levels: >12 mcg/mL • Timing: before morning dose • Time to Steady State: >1mo • Other common monitoring parameters: BUN/Cr, CBC w/ diff, LFTs, urinalysis, ophthal. exams at baseline, then periodically; serum drug levels; s/sx depression, behavior changes, suicidality Oxcarbazepine Oxcarbazepine Adverse Effects • Common : Dizziness (30-50%), Diplopia (30-50%), Headache (26-30%), Nausea/vomiting (26-30%), Nystagmus (26-30%), Somnolence (26-30%), Ataxia (10-30%), Abnormal gait (16-20%), Tremor (16-20%), Abdominal pain (11-15%), Fatigue (11-15%), Vertigo (11-15%), Vision abnormalities (11-15%) • Less Common: Dyspepsia (5-6%), Rash (4%), Insomnia (2-4%), Abnormal thinking (<4%), Hyponatremia (1-3%), Muscle weakness (1-2%), Hypotension (<2%), Speech disorder (1%), Asthenia • Post-marketing reports: Angioedema, Anaphylaxis, Bone marrow depression, agranulocytosis, aplastic anemia, pancytopenia, neutropenia, Pancreatitis and/or lipase and/or amylase increased, Folic acid deficiency, hypothyroidism, Erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, Multiorgan hypersensitivity disorders characterized by features such as rash, fever, lymphadenopathy, abnormal liver function tests, eosinophilia, and arthralgia • Pregnancy Category: C Oxcarbazepine Drug Interactions • • • • • CYP2C19 inhibitor, moderate CYP3A4 inducer, minor CNS depression Hyponatremia Serotonergic effects, weak Oxcarbazepine Dosing • 300 mg/day PO initially; may titrate to 1800-2400 mg/day maximum Oxcarbazepine Monitoring • No specific target plasma concentration recommendations • Other common monitoring parameters: Cr at baseline; Na; s/sx depression, behavior changes, suicidality Mood Stabilizer Costs Drug Average Cost* Lithium $9 W Lithobid (lithium er) $18 Depakote (divalproex) $20 Depakote ER (divalproex er) $105 Lamotrigine $9 Lamictal XR (lamotrigine er) $186 Carbamazepine $6 W Equetro (carbamazepine) $72 oxcarbazepine $27 *GoodRx.com price comparison; W=Walmart $4/$10 generic STEP-BD NIMH-funded Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) is a long-term outpatient study designed to find out which treatments, or combinations of treatments, are most effective for treating episodes of depression and mania and for preventing recurrent episodes in people with bipolar disorder. STEP-BD • N= 4360 total (Bipolar I, II, NOS, cyclothymia); assigned to “STEP-BDcertified psychiatrist” • Major findings: • After up to two years of best practices treatment, 58.4% of patients met criteria for full recovery. 48.5% had a recurrence (72% with a depressive episode; 28% with a manic, hypomanic, or mixed episode). Residual sx was assoc with an ↑chance of recurrence. • Adjunctive psychosocial treatments but not adjunctive antidepressants yielded outcomes superior to those achieved with mood stabilizers alone in bipolar depression. www.nimi.nih.gov; CNS Neuroscience & Therapeutics 2012; 18(3): 253-9; Am J Psychiatry 2008; 165:370-377 STEP-BD • Major findings: • Lamotrigine appears to be more effective than risperidone in treatmentrefractory patients with bipolar depression . Study assigned pts to one of three adjunctive treatments: lamotrigine (up to 250 mg QD), inositol (up to 25 g QD), or risperidone (up to 6 mg QD) for up to 16 weeks. The recovery rates were: lamotrigine, 23.8%; inositol, 17.4%; risperidone, 4.6% (findings not ss). • Valproate is associated with PCOS. Study evaluated 230 women (ages 18- 44) for the development of PCOS sx (menstrual irregularities, hirsutism, acne, hair loss, ↑testosterone). They found PCOS symptoms in 9 of 86 women on valproate (10.5%) compared to only 2 of 144 women on a nonvalproate anticonvulsant or lithium (1.4%). • Prevention of rapid cycling recurrences may require early intervention and restricted use of antidepressants. www.nimi.nih.gov; CNS Neuroscience & Therapeutics 2012; 18(3): 253-9; Am J Psychiatry 2008; 165:370-377 The End! Next Week: Looking forward to Dr. Betlinski’s return