* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ravimid laste jaoks
Pharmacognosy wikipedia , lookup
Clinical trial wikipedia , lookup
Orphan drug wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
Prescription costs wikipedia , lookup
Theralizumab wikipedia , lookup
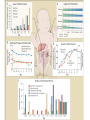
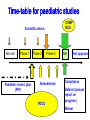
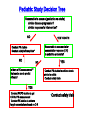
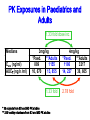
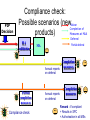
Drugs for children Irja Lutsar Tallinn 2007 Pediatrics does not deal with miniature men and women, with reduced doses the same class of disease in smaller bodies, but ….. has its own independent range and horizon Abraham Jacobi rohkem kui 100 aastat tagasi Problem statement: child vs adult • The effectiveness and tolerabily is likely to be different – – – – Neonates (premature and full-term) - < 28 days Infants – 1 mo - < 2years Children – 2 years – 11 years Adolescents – 12 years – 16 (-17) years • Dose cannot be directly extrapolated from adults – Different pharmacokinetics • absorption • distribution • elimination • Many agents used in paediatric are never tested in children Pharmacokinetics of pencillin G Premature < 1200 g 30 mg/kg 15 mg/kg Full-term baby 15 mg/kg adult 600 mg/dosi T1/2 Cmax 3,8 h 4,6 h 145,5 mcg/ml 58,9 mcg/ml 3,4 h 22,0 mcg/ml 0,5 h 45 mcg/ml Metsvaht et al. AAC 2007; 51: 1995-00 Mean voriconazole plasma concentrations after IV and POS administration Mean plasma voriconazole concentration (ng/mL) BA in adults 96% upper limit of SD: 19,030 12000 10000 8000 6000 4000 2000 0 0 2 4 6 8 10 12 Time postdose (h) 6 mg/kg IV (120 min infusion) Walsh et al. AAC 2007 8 mg/kg IV (160 min infusion) 6 mg/kg POS Formulations not suitable for children • IV formulation toxic or too concentrated – Cyclodextran & nephrotoxic – Quinolones are reconstituted in acids – Not suitable presentations (too large or too small vials) • Oral formulations – Capsules and film-coated tablets (children cannot swallow) – Crushed tablets vs whole tablets – How to give bitter tablets? – Solution and suspensions • • • • • Better solubility worse taste What is a good taste? Should a medicine have a good taste? Too large amounts Bioavailability Slow release tablet Crushed tablet History • 1937 – 107 people with streptococcal infection died (mainly children) – All were treated with a sulphonamide that was diluted in diethylglycole (antifris) • 1956 – neonates who received sulphonamides had more often kernicterus than those receiving tetracycline • Chloramphenicol & “gray baby syndrome” (enzyme immaturity) • “Gasping syndrome” – Agents that are reconstituted in benzylalcochol (IV clindamycin) • Antidepressants How common is off-label use Years >1 unlicenced or off-label drug (%) off-label (%) Children (hospitalised) 1985-99 36-92 7-60 Neonates (hospitalised) 1998-02 80-97 14-63 Outpatient 1978-01 16-56 9-33 Then younger the child then lower number of studies Then sicker the child the less studies Eur J Pediatr 2005; 164: 552-558 Studies in children: statistics • 1997 – FDA 33 new drugs licenced – 27 potentially used for children – 9 had some information on use in children • Neonates use a lot of medicines – Detroit (USA) 1997-2004 NICU 3,6/per child – ELBW - 11,7/per child – Australia 93% ELBW at least 1 untested drug will be used FDA: paediatric studies • FDAMA Pediatric Exclusivity 1997 (voluntary) • Pediatric Rule Regulation 1998 (enjoined 2002) • January 2002 - FDAMA Exclusivity Sunsets • January 2002 (compulsary) - Best Pharmaceuticals for Children Act (BPCA) • December 2003 - Pediatric Research Equity Act (PREA) • October 2007: Sunset for BPCA & PREA FDA: stick (WR) & carrot (PE) • All new drugs ned to be tested in children • Industry initiated (WR) • waivers • Patent prodection for 6 months – Fluconazole 600 mill $ – Sildenafil 1 bill $ – Disease does not exsist in children – Drug is too toxic for children • Deferral until more data on adults are available Priotrity list of medicines Pediatric Drug Development Number 120 69 60 98 45 21 24 12 19 15 19 10 22 25 25 14 11 3 12 17 23 20 23 17 15 18 1999 2000 2001 2002 2003 2004 2005 2006 0 Calendar year Pediatric exclusivity determinations Written requests issued Pediatric exclusivity labeling changes FDA: results (March 2007) • FDA issued 341 WR • 150 new medicines were tested in children • 128 labelling changes were made Benefits of WR (FDA) • 1998- 2004 – 253 studies in children with new agents – – – – 125 (50%) – efficacy 51 (20%) – MD PK 34 (13%) – SD PK 43 (17%) – safety & tolerability • 127 (50%) new information • 113 (45%) published • Net economic return - -8,9 mil....507.9 mil $ JAMA 2006; 296: 120-123 JAMA 2007; 297: 480-8 FDA: 127 changes in drug information • New dose or changes in exsisting dose (n = 25) • New data on tolerability (n = 35) • “No data in children” added into label (n = 28) • New dosing recommendations for infants or neonates (n = 83) We got know what we do not know Many questions were answered but many mopre questions raised Deficiencies in the FDA regulations • Industry might not be interested – Pediatric market is small – Studies in children are complicated – Long-lasting negotiations • No procedure to study off patent drugs • No paediatric trial registry European Union The same issues Paediatric regulation: entered into force 26 January 2007 • Improve the health of children – Increase high quality, ethical research into medicines for children – Increase availability of authorised medicines for children – Increase information on medicines • Achieve the above – Without unnecessary studies in children – Without delaying authorisation for adults Main pillars of the Regulation • New EMEA Committee: the Paediatric Committee • An agreed (evolutive) paediatric development: the Paediatric Investigation Plan (PIP) • A mix of rewards and incentives – For on-patent products – For off-patent products • A series of other tools for information, transparency, and stimulation of research Paediatric Committee (PDCO) to be established by 26 July 2007 + alternates Paediatric committe: objectives • Paediatric scientific advice – Free of charge – Not binding • Review of PIPs (+waivers & deferrals) • Member States Survey of all existing uses of medicinal products in children, including off-label within 2 years, final EMEA inventory in third year (2009) • Update of Paediatric needs by Paediatric Committee on basis of inventory Currently unauthorised products 18 months after entry into force of the Regulation, 26.07 2008 • Stick – Obligation to submit results of agreed PIP at time of marketing authorisation application – If not: Invalid application for MA – Results reported in SmPC – Authorisation in all Member States • Carrot – 6-month extension of the Supplementary Protection Certificate Patent-protected authorised products 24 months after entry into force of the Regulation, 26.01 2009 • Stick – Obligation to submit results of agreed PIP at time of change (variation/extension) for new indication, route of administration, or pharmaceutical form – Results reported in SmPC – Authorisation in all Member States • Carrot – 6-month extension of the Supplementary Protection Certificate For off-patent products • Paediatric Use Marketing Authorisation (PUMA) New type of MA • Covers exclusively paediatric indication(s) and formulation(s) • Optional but need for PIP and compliance • No need for MA in all Member States • Brand name may be retained Carrot • 10 years of data protection: (8+2) +1 Paediatric Investigation Plan • General strategy of paediatric studies – Epidemiology, pathophysiology, indications, target population, doses • New formulations (needs & technology) • Preclinical studies (toxicity, effect on pregnancy, young animals) • Clinical studies (PK studies, efficacy/safety studies, strategy, time-table) • Waivers and deferrals Time-table for paediatric studies CHMP NCA Scientific advice Non-clin Phase 1 Phase 2 Phase 3 MA Post approval 1 Paediatric invest. plan (PIP) Amendments PDCO Compliance Deferral (annual report on progress) Waiver Waivers • Product likely to be ineffective or unsafe in all or part of the paediatric population • Disease occurs only in adults • No significant therapeutic benefit over existing treatments for children → for one or more subgroups of the paediatric population → for one or more specified indications Deferrals • Request to defer initiation or completion of some or all the measures set out in the PIP • On initiative from applicant or Committee • For all or part of Paediatric Investigation Plan • Annual report to monitor deferred studies A European Network EMEA paediatric research network • To link together existing networks, investigators and centres with specific paediatric expertise • Build up competences at a European level • Facilitate the conduct of studies • Avoid duplication of studies Preparatory work at EMEA over 2005-6 Paediatric studies are not the private business of the pharmaceutical industry Types of paediatric studies • Direct clinical studies – PK studies – Efficacy and toleration studies – Preclinical and non-clinical studies • Indirect studies – Assessment of paediatric standards • Growth and development – Epidemiological studies • Prevalence of the disease in children • Pathomechanism of the disease – Investigations on growth, development & maturation Who should conduct studies in children • Pharmaceutical industry • Universities, research institutes, medical schools • All paediatricians – why not? Who should fund paediatric studies • Pharmaceutical industry • Pharmaceutical industry + private funds • EU governmental funds (research councils, science boards) • EU grants – EU 7FP specific call for paediatric studies for offpatent used drugs – Priority list • Paediatric Societies • Charities – Bill & Melinda Gates foundation Other Measures: Funding of studies into off-patent medicines for children • Community funding • Liaison with EC (DG Research) for calls for proposals under the 7th framework programme (special programme for off-patent drugs) • Priority list of off-patent medicinal products for paediatric studies (published in December 2006 for comments till 31 January 2007- Finalisation at the March Paediatric Working Party) Paediatric studies – how to start? Questions to answer first? • Does the disease occur in paediatrics? • How common is the disease in paediatrics? – Common → continue – Rare → ??? • Is there an unmet medical need? – Yes → continue • Which are the potential paediatric indications? – Similar to adults – Different of adults • Is the disaese process in children and adults similar? – – – – Infections – yes Fever – yes Seizures – unknown HIV - no Planning is the key • Which studies when and how • Non-clinical studies (toxicity, juvenile animals) • Formulation issues –child friendly – suspension, solution – Taste and amount – IV formulation (concentration??) • Veins are narrow • Limited amount of fluid could be given – Bioavailability • Safety/efficacy in all paediatric populations Pediatric Study Decision Tree Reasonable to assume (pediatrics vs adults) similar disease progression? similar response to intervention? NO YES TO BOTH Reasonable to assume similar concentration-response (C-R) in pediatrics and adults? •Conduct PK studies •Conduct safety/efficacy trials* NO NO Is there a PD measurement** that can be use to predict efficacy? YES •Conduct PK studies to achieve levels similar to adults •Conduct safety trials YES •Conduct PK/PD studies to get C-R for PD measurement •Conduct PK studies to achieve target concentrations based on C-R •Conduct safety trials An ideal drug • PK established in all paediatric populations • Safety well described • Efficacy can be extrapolated from adults • Child-friendly formulation available • Post-marketing experience • No need for further paediatric studies • Studies with new formulation could be performed in adults Unmet medical need & some paediatric data • Pharmacokinetic studies – Linear pharmacokinetics – SD studies – Non-linear pharmacokinetics – MD studies – Target concentration • Safety studies if efficacy can be extrapolated from adults • Efficacy studies if efficacy cannot be extrapolated from adults • Step-down timing – Studies in adults and in older children first • Do not forget neonates, if there is a need PK Exposures in Paediatrics and Adults 1.33 fold dose inc. Medians Cave (ng/ml) AUC (ngh /ml) 3mg/kg *Paed. **Adults 889 1155 10, 670 13, 855 1.33 fold * 35 subjects from SD and MD PK studies ** 236 healthy volunteers from SD and MD PK studies 4mg/kg *Paed. **Adults 1186 3217 14, 227 38, 605 2.78 fold Ethical issues • Is it ethical to conduct studies in children? • Is it ethical not to conduct studies in children? • Points to consider – – – – – – – Sample size Pain and dyscomfort Informed consent and assent Blood loss No investigations for study purposes only Effect on growth and development Is there a benefit (for this child, for the group of children with similar disease) – Healthy volunteers are not accepted Kuidas Eesti saaks osaleda laste uuringutes • Kui palju Eestis kasutatavaid ravimeid omab andmeid kõigi eagruppide kohta? • Uuringute register lastearstide seltsi juures – Farmaatsiatööstuse poolt läbiviidavad uuringud pole ainukesed kliinilised uuringud lastel • • • • EU grandid koos kolleegidega Euroopast EV valitsus - ETF, sihtfinatseerimine Heategevuslikud fondid (nt. Lastefond jne) Rahva teavitamine – Miks ja kuidas teostatakse uuringuid lastel Summary • Political decision - there is a need for more paeditaric data • Each day new paediatric data will be released • Future - All medicines used in children should have paediatric data Pediaatrilised uuringud on globaalsed • Luua sidemed 3-ndate riikide andmebaasidega • Lasteuuringute andmebaas kõigile veebis kättesaadavaks PIP Decision Compliance check: Possible scenarios (newWaiver Completion of Measures at MAA products) Deferral MA validation Partial deferral MA Completion measures Annual reports on deferral Partial completion measures Compliance check Annual reports on deferral Completion measures Reward : If compliant + Results in SPC + Authorisation in all MSs • Other Measures: Paediatric Scientific Free of charge fromAdvice entry into force • Prior to submission of a PIP or during PIP implementation process • Including advice on pharmacovigilance and risk management systems • Not binding on Paediatric Committee • Link Paediatric Committee / Scientific Advice Working Party Other Measures: Provision of Information Paediatric clinical trials requiring EUDRACT Modification To include third countries trials linked to a PIP To include results of all trials and of other trials ‘submitted to competent authorities’ Paediatric information to be made public Proposal made by EMEA to EC on the data fields to be included in EudraCT and those to be published on paediatric medicines For discussion at the Ad Hoc Group for the development of implementing guidelines for Directive 2001/20/EC Followed by Release for consultation Public access to paediatric information for authorised New EMEA Committee: the Paediatric Committee • An agreed (evolutive) paediatric development: the Paediatric Investigation Plan (PIP) • A mix of rewards and incentives – For on-patent products – For off-patent products • A series of other tools for information, transparency, and stimulation of research • • • • • • • • • • New EMEA Committee: the Paediatric Committee • An agreed (evolutive) paediatric development: the Paediatric Investigation Plan (PIP) • A mix of rewards and incentives – For on-patent products – For off-patent products • A series of other tools for information, transparency, and stimulation of research BPCA: Pediatric Exclusivity Stats (As of December 2006) • Number of patients requested for studies N= 45,700 • Products with Safety Reviews Presented to PAC N= 65 http://www.fda.gov/oc/opt/pediatricsafety.html • Summaries of Medical/Clinical Pharmacology – Summaries on fda.gov/cder/pediatrics N=73 – www.fda.gov/cder/pediatric/summaryreview.htm BPCA: Pediatric Exclusivity Stats (As of December 2006) • 65 products had safety reviews presented to Pediatric Advisory Comm. • 73 summaries of Medical/Clinical Pharmacology posted on web. • 56 products placed on the BPCA offpatent priority list BPCA vs. PREA • • • • • • • • BPCA Studies are voluntary Includes orphan drugs and orphan drug indications Drugs only Studies on whole moiety 1 year safety reviews Summary posted on Web Central review=PDIT 10-1-07 Sunset • • • • • • • PREA Studies are required Orphan drugs designated exempt Biologics and Drugs Studies limited to drug/indication under development No focused postmarketing safety review No central review 10-1-07 Sunset BPCA: Before a Written Request (WR) is issued, we answer these questions. • Public Health Benefit – yes • Risk/Benefit appropriate -- yes Then we ask … • What information do we need? • In what age groups do we need the information? • What studies are needed to obtain this information Process: BPCA 1. Sponsor makes proposal for WR. Division rejects, accepts, or modifies. OR 2. Division develops WR independent of sponsor 3. Division presents WR to PDIT(Pediatric Team) 4. Office Director signs off on WR to sponsor 5. Sponsor accepts or declines. 6. If sponsor declines, may be sent to FNIH 7. There is tracking of applications submitted for an exclusivity determination 8. A summary of the studies is posted for all studies 9. There is a 1 year post exclusivity safety review Process: PREA 1. Sponsor submits an IND for an adult indication 2. Division must decide if pediatric studies are needed and if they can be deferred or if pediatric studies can be waived. 3. If studies are required the time table and general outline are decided before an action is taken on the application. 4. There may or may not be involvement of the pediatric staff. There is no central process. 5. There is no tracking of outcomes except as Phase 4 6. Only approved applications have studies posted 7. There is no mandatory post approval safety Selected Pediatric Ethics Activities • Pediatric Ethical Consults – Sept 2003 – July 2006: approx. 80 consults – Topics: pediatric safety, compliance with Subpart D, parental permission and child assent, exception from informed consent applied to parents, use of “healthy” children, international studies • Subpart D (additional protections for children) Referrals under 21CFR§50.54 – Pediatric Ethics Subcommittee of Pediatric AC: FACA review of protocols referred by local IRBs for approval by FDA Commissioner and/or HHS Secretary – Since 2004: 3 reviews (prior OHRP non-FACA reviews=14) Subpart D Pediatric Advisory Committee and Ethics Subcommittee meetings • September 2004: Effects of single dose Dextroamphetamine in ADHD and functional imaging • June 2005: Precursor Preference in Surfactant Synthesis of Newborns • November 2005: Gonadotropin Releasing Hormone Agonist Test in Disorders of Puberty