* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Merging Marketing and Clinical
Survey
Document related concepts
Transcript
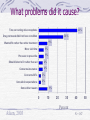
Regulatory & Public Health Implications of DTCA Economists Conference Louis A. Morris, Ph.D. April 29, 2003 AARP Bulletin, March 2002 AARP Bulletin, March 2002 March 13, 2002 March 14, 2002 More Than Ads, Drug Makers Rely on Sales Representatives Backlash Rises Against Flashy Ads For Prescription Pharmaceuticals Ad Agencies Begin to Participate FDA Is Inundated Trying In Development of New Drugs To Assess Drug Ad Pitches European Drug Makers May Utilize More Aggressive Marketing Tactics FD&C Act - 1962 Amendments Advertisements and Labeling must contain a true statement of the drug’s intended uses cannot be false or misleading must contain a brief summary Regulations fair balance contextual overall DTC Policy “Change” Draft Guidance - July, 1997 comment period closed October, 1997 Adequate Provision toll free telephone concurrent print or other availability HCP (MD, RPh, Vet.) provide information Internet Presumes Major Statement FDA Letters: 8 Pitfalls of DTCA Reminder/Institutional Implied Claims Disclosure Adequacy Contextual Fair Balance Limits on Effectiveness Overall Fair Balance Unsubstantiated Claims Distractions “RID the CLOUD” 2002 DDMAC Letters N = 27 DTC Objections Medical Meeting 9 5 Oral Statements Oral Statements at Hosp Outcomes Claims Web – Pre-approval 3 1 2 1 Down from over 100 letters in previous years Seems like the same mix of topics 2002 DTC Ads Media TV/Radio Print Telephone 5 4 1 FDA Objection Lack of Risk Info Misleading Implications Fact-Claim Discrepancy Not a Reminder 3 4 3 2 Lots of claim interpretation objections for DTC March 31, 2003 Ads Boost Consumer Awareness Of Drugs, but Downplay Risks . Drug makers spent almost $3 billion on consumer ads last year, about a 7% increase over 2001. And consumers generally like them, notwithstanding some suspicions that the ads inflate drug costs, surveys show. But doctors, regulators and consumer advocates continue to have concerns. A survey of 500 doctors conducted by the FDA last year and presented at the conference asked whether consumer drug ads confused patients about the relative risks and benefits of medicines. Some 70% of general practitioners and 60% of specialists answered "somewhat" or "a great deal." Only 5% of general practitioners and 10% of specialist said "not at all." DTC April 15, 2002 ADVERTISING Patients' Success in Drug Requests Shows the Power of Medication Ads Beneficial Effects – MD Survey YES 41% NO 59% Did the fact that this patient saw an advertisement have any beneficial effects for your interaction with this patient? Aiken, 2003 N = 459 What beneficial effects did it have? 53% Better discussion with patient 42% Patient more aware of treatments Informs/educates 10% Patient more likely to take prescribed drug 10% 9% Patient more likely to consider using Rx drug 6% New condition was discovered 2% Patient sought treatment for serious condition 3% Some other reason 0 10 20 30 40 50 Percent Aiken, 2003 N = 187 60 Problems YES 18% NO 82% Did the fact that this patient saw an advertisement create any problems for your interaction with this patient? Aiken, 2003 N = 459 What problems did it cause? Time correcting misconceptions 41% 26% Drug not needed/did not have condition 9% Wanted Rx rather than other treatment More visit time 5% Pressure to prescribe 5% Should listen to Dr rather than ad 4% Concerns-insurance 4% Concerns-SE's 2% Unrealistic expectations 2% 9% Some other reason 0 Aiken, 2003 10 20 30 40 Percent N = 187 50 Current Views of DTC Surveys show few Benefits, few Problems Risk Communication – balance a key issue Suggestion to pay into consumer education “pot” DTC remains heavily regulated Implied Claims often focus of objection FDA regulation is mitigated by GC review Doctors still skeptical Still despised by MCOs and payers Risk Management Issue Forteo (Lilly), no DTC