* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 20130912Canagliflozin&Linagliptin
Survey
Document related concepts
Transcript
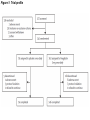
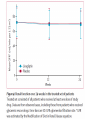
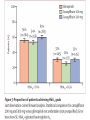
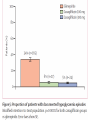
Journal Club Barnett AH, Huisman H, Jones R, von Eynatten M, Patel S, Woerle HJ. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebocontrolled trial. Lancet. 2013 Aug 12. doi:pii: S0140-6736(13)61500-7. 10.1016/S0140-6736(13)61500-7. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013 Jul 11. pii: S0140-6736(13)60683-2. doi: 10.1016/S0140-6736(13)60683-2. 2013年9月12日 8:30-8:55 8階 医局 埼玉医科大学 総合医療センター 内分泌・糖尿病内科 Department of Endocrinology and Diabetes, Saitama Medical Center, Saitama Medical University 吉永 玲恵 松田 昌文 Yoshinaga, Ree, Matsuda, Masafumi Diabetes Centre, Heart of England NHS Foundation Trust, Birmingham, UK (Prof A H Barnett MD); University of Birmingham, Birmingham, UK (Prof A H Barnett); Boehringer Ingelheim, Alkmaar, Netherlands (H Huisman MSc); Boehringer Ingelheim, Bracknell, UK (R Jones MSc, S Patel MB ChB); and Boehringer Ingelheim, Ingelheim, Germany (M von Eynatten MD, H-J Woerle MD) Lancet. 2013 Aug 12. doi:pii: S0140-6736(13)61500-7. 10.1016/S0140-6736(13)61500-7. Background A substantial proportion of patients with type 2 diabetes are elderly (≥65 years) but this group has been largely excluded from clinical studies of glucose-lowering drugs. We aimed to assess the effectiveness of linagliptin, a dipeptidyl peptidase-4 inhibitor, in elderly patients with type 2 diabetes. Methods In this randomised, double-blind, parallel-group, multinational phase 3 study, patients aged 70 years or older with type 2 diabetes, glycated haemoglobin A1c (HbA1c) of 7・0% or more, receiving metformin, sulfonylureas, or basal insulin, or combinations of these drugs, were randomised (by computer-generated randomisation sequence, concealed with a voice– response system, stratified by HbA1c level [<8・5% vs ≥8・5%] and insulin use [yes vs no], block size four) in a 2:1 ratio to once-daily oral treatment with linagliptin 5 mg or matching placebo for 24 weeks. Investigators and participants were masked to assignment throughout the study. The primary endpoint was change in HbA1c from baseline to week 24. This trial is registered with ClinicalTrials.gov, number NCT01084005. Figure 1: Trial profile Table 1: Baseline demographics and clinical characteristics in the treated set of patients FPG=fasting plasma glucose. GFR=glomerular filtration rate. HbA1c=glycated haemoglobin A1c. Figure 2: Baseline disease-related characteristics and comorbidities in the treated set of patients Treated set consisted of all patients who received at least one dose of study drug. *Renal function according to GFR estimated by the Modifi cation of Diet in Renal Disease equation. †Microvascular disease consisted of diabetic retinopathy, nephropathy, and neuropathy. ‡Macrovascular disease consisted of coronary art ery disease, peripheral artery disease, cerebrovascular disease, and hypertension. §Sulfonylureas, or meglitinides, or insulin, or combination thereof. ¶Drug in addition to glucose-lowering drugs. IIGlucose-lowering drugs and other drugs. Figure 2: Charlson age-comorbidity score in the treated set of patients Treated set consisted of all patients who received at least one dose of study drug. Charlson M, Szatrowski TP, Peterso n J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–51. The mean Charlson age-comorbidity score of more than 5 suggests a highrisk population. The Charlson comorbidity index predicts the ten-year mortality for a patient who may have a range of comorbid conditions, such as heart disease, AIDS, or cancer (a total of 22 conditions). Each condition is assigned a score of 1, 2, 3, or 6, depending on the risk of dying associated with each one. Scores are summed to provide a total score to predict mortality. Many variations of the Charlson comorbidity index have been presented, including the Charlson/Deyo, Charlson/Romano, Charlson/Manitoba, and Charlson/D'Hoores comorbidity indices. Clinical conditions and associated scores are as follows: 1 each: Myocardial infarct, congestive heart failure, peripheral vascular disease, dementia, cerebrovascular disease, chronic lung disease, connective tissue disease, ulcer, chronic liver disease, diabetes. 2 each: Hemiplegia, moderate or severe kidney disease, diabetes with end organ damage, tumor, leukemia, lymphoma. 3 each: Moderate or severe liver disease. 6 each: Malignant tumor, metastasis, AIDS. For a physician, this score is helpful in deciding how aggressively to treat a condition. For example, a patient may have cancer with comorbid heart disease and diabetes. These comorbidities may be so severe that the costs and risks of cancer treatment would outweigh its short-term benefit. Since patients often do not know how severe their conditions are, nurses were originally supposed to review a patient's chart and determine whether a particular condition was present in order to calculate the index. Subsequent studies have adapted the comorbidity index into a questionnaire for patients. Table 2: Change from baseline in HbA1c and FPG after 24 weeks in the full analysis set of patients, last observation carried forward Figure 3: Change from baseline in HbA1c over 24 weeks in the full analysis set Adjusted mean changes in HbA1c. Error bars are SE. Full analysis set consisted of all randomised patients who received at least one dose of study drug, and who had a baseline and at least one on-treatment HbA1c measurement. Data are from a mixed model for repeated measurements, using observed cases with treatment, visit, previous use of insulin, and visit by treatment interaction as fixed classification effects, and baseline HbA1c as a linear covariate. HbA1c=glycated haemoglobin A1c. Table 4: Summary of adverse events during 24 weeks in the treated set of patients not receiving concomitant sulfonylurea treatment Data are n (%). Treated set consisted of all patients who received at least one dose of study drug. CEC=clinical endpoint committee. *Deemed to be related to study drug by the investigator. †Hypersensitivity reaction, renal adverse event, hepatic adverse event. ‡Preferred terms from the Medical Dictionary for Regulatory Activities (version 14.1). Findings 241 community-living outpatients were randomised (162 linagliptin, 79 placebo). Mean age was 74・9 years (SD 4・3). Mean HbA1c was 7・8% (SD 0・8). At week 24, placeboadjusted mean change in HbA1c with linagliptin was −0・64% (95% CI −0・81 to −0・48, p<0・0001). Overall safety and tolerability were much the same between the linagliptin and placebo groups; 75・9% of patients in both groups had an adverse event (linagliptin n=123, placebo n=60). No deaths occurred. Serious adverse events occurred in 8・6% (14) of patients in the linagliptin group and 6・3% (fi ve) patients in the placebo group; none were deemed related to study drug. Hypoglycaemia was the most common adverse event in both groups, but did not differ between groups (24・1% [39] in the linagliptin group, 16・5% [13] in the placebo group; odds ratio 1・ 58, 95% CI 0・78–3・78, p=0・2083). Interpretation In elderly patients with type 2 diabetes linagliptin was efficacious in lowering glucose with a safety profile similar to placebo. These findings could inform treatment decisions for achieving individualised glycaemic goals with minimal risk in this important population of patients. Funding Boehringer Ingelheim. Message メトホルミンやスルホニル尿素薬などで血糖コ ントロール不良の70歳以上の2型糖尿病(DM)患 者241人を対象に、リナグリプチンの有効性を無 作為化プラセボ対照試験で検討。リナグリプチ ン群におけるHbA1c値のベースラインからのプラ セボ調整後平均変化量は-0.64%だった(P< 0.0001)。安全性と忍容性は両群で同等だった。 Diamant M, Morsink LM.: SGLT2 inhibitors for diabetes: turning symptoms into therapy. Lancet. 2013 Jul 11. doi:pii: S0140-6736(13)60902-2. 10.1016/S0140-6736(13)60902-2. [Epub ahead of print] Pennington Biomedical Research Center, Baton Rouge, LA, USA (Prof W T Cefalu MD); Louisiana State University Health Sciences Centre School of Medicine, New Orleans, LA, USA (Prof W T Cefalu); Keenan Research Centre, Li Ka Shing Knowledge Institute, St Michael’s Hospital, University of Toronto, Toronto, ON, Canada (Prof L A Leiter MD); The Catholic University of Korea, Seoul St Mary’s Hospital, Seoul, South Korea (Prof K-H Yoon MD); University of Rosario Medical School, Rosario, Argentina (Prof P Arias MD); Litoral University Medical School, Santa Fe, Argentina (Prof P Arias); University of Eastern Finland, Kuopio, Finland (Prof L Niskanen MD); and Janssen Research & Development, LLC, Raritan, NJ, USA (J Xie PhD, D A Balis PharmD, W Canovatchel MD, G Meininger MD) Lancet. 2013 Jul 11. pii: S0140-6736(13)60683-2. doi: 10.1016/S0140-6736(13)60683-2. Background Sodium–glucose cotransporter 2 (SGLT2) inhibitors improve glycaemia in patients with type 2 diabetes by enhancing urinary glucose excretion. We compared the efficacy and safety of canagliflozin, an SGLT2 inhibitor, with glimepiride in patients with type 2 diabetes inadequately controlled with metformin. Methods We undertook this 52 week, randomised, double-blind, activecontrolled, phase 3 non-inferiority trial at 157 centres in 19 countries between Aug 28, 2009, and Dec 21, 2011. Patients aged 18–80 years with type 2 diabetes and glycated haemoglobin A1c (HbA1c) of 7・0–9・5% on stable metformin were randomly assigned (1:1:1) by computergenerated random sequence via an interactive voice or web response system to receive canagliflozin 100 mg or 300 mg, or glimepiride (up-titrated to 6 mg or 8 mg per day) orally once daily. Patients, study investigators, and local sponsor personnel were masked to treatment. The primary endpoint was change in HbA1c from baseline to week 52, with a non-inferiority margin of 0・3% for the comparison of each canagliflozin dose with glimepiride. If noninferiority was shown, we assessed superiority on the basis of an upper bound of the 95% CI for the difference of each canagliflozin dose versus glimepiride of less than 0・0%. Analysis was done in a modified intention-to-treat population, including all randomised patients who received at least one dose of study drug. This study is registered with ClinicalTrials.gov, number NCT00968812. Figure 1: Trial profile eGFR=estimated glomerular filtration rate. *484 patients randomly assigned. Figure 2: Change in HbA1c (A), and mean HbA1c over time (B) Last observation carried forward analyses. Mean baseline HbA1c of 7・ 8% for each treatment group. LS=least squares. HbA1c=glycated haemoglobin A1c. Table 4: Changes from baseline in blood pressure, pulse rate, fasting plasma lipids, and fasting insulin at week 52 Conversion factor Cholesterol 0.0259 Triglyceride 0.0113 168(106) 104(35) 186(133) 100 (35) 186 (186) mg/dl 108(35) mg/dl Increased! 46(12) 46 (12) 46(12) mg/dl not different! Last observation carried forward analyses. Statistical comparison for canagliflozin 100 mg and 300 mg versus glimepiride not undertaken (not prespecified). LS=least squares. 135(39) 135 (39) 143(42) mg/dl Conversion factor Insulin pmol/L to microunits/mL 7.18 9.9(10.0) 9.6 (7.0) 9.0(6.1) mU/ml Table 5: Overall safety and selected adverse events Table 6: Summary of laboratory parameters at baseline and week 52 We recorded no notable differences in serum electrolytes, sodium and potassium, with canagliflozin compared with glimepiride (data not shown). Statistical comparison for canagliflozin 100 mg and 300 mg versus glimepiride not undertaken (not prespecified). ALT=alanine aminotransferase. AST=aspartate aminotransferase. BUN=blood urea nitrogen. GGT=gamma-glutamyltransferase. Findings 1450 of 1452 randomised patients received at least one dose of glimepiride (n=482), canagliflozin 100 mg (n=483), or canagliflozin 300 mg (n=485). For lowering of HbA1c at 52 weeks, canagliflozin 100 mg was non-inferior to glimepiride (least-squares mean difference –0・01% [95% CI –0・11 to 0・09]), and canaglifl ozin 300 mg was superior to glimepiride (–0・12% [–0・22 to –0・02]). 39 (8%) patients had serious adverse events in the glimepiride group versus 24 (5%) in the canagliflozin 100 mg group and 26 (5%) in the 300 mg group. In the canagliflozin 100 mg and 300 mg groups versus the glimepiride group, we recorded a greater number of genital mycotic infections (women: 26 [11%] and 34 [14%] vs five [2%]; men: 17 [7%] and 20 [8%] vs three [1%]), urinary tract infections (31 [6%] for both canagliflozin doses vs 22 [5%]), and osmotic diuresis-related events (pollakiuria: 12 [3%] for both doses vs one [<1%]; polyuria: four [<1%] for both doses vs two [<1%]). Interpretation Canaglifl ozin provides greater HbA1c reduction than does glimepiride, and is well tolerated in patients with type 2 diabetes receiving metformin. These fi ndings support the use of canaglifl ozin as a viable treatment option for patients who do not achieve suffi cient glycaemic control with metformin therapy. Funding Janssen Research & Development, LLC. Message CANTATA-SU試験は、メトホルミン単独では血糖値の管理が十分で ない2型糖尿病患者において、カナグリフロジンの上乗せ効果の、 グリメピリド(商品名:アマリールほか)に対する非劣性を検証 する二重盲検無作為化第III相試験。カナグリフロジン 100mgま たは300mg、あるいはグリメピリド(6から8mgへ漸増)を1日1回 経口投与する群に無作為化に割り付けられた。グリメピリド群に 482例、カナグリフロジン 100mg群に483例、300mg群には485例が 割り付けられた。治療52週時のHbA1cは3群ともにベースラインよ りも低下し、最小二乗平均値の変化率はグリメピリド群が-0.81、 カナグリフロジン 100mg群は-0.82%、300mg群は-0.93%で あった。体重は、グリメピリド群がわずかに増加したのに対し、 2つのカナグリフロジン群は有意に低下した。尿路感染症も100mg 群が31例(6%)、300mg群が31例(6%)で、グリメピリド群の 22例(5%)より多い傾向がみられた。 糖質食べても尿から出すからOK!というアメリカで大人気のお薬 SGLT2阻害薬追加、血糖管理不良な2型糖尿病に有用 ナトリウム/グルコース共輸送体(SGLT)2阻害薬カナグリフロジン(canagliflozin)は、メトホルミン単独では血糖管理が十分でない2型糖尿 病患者に対する追加治療として良好な血糖改善効果を発揮し、忍容性も良好なことが、米国・ペニントン生物医学研究所のWilliam T Cefalu 氏らが行ったCANTATA-SU試験で示された。メトホルミンへの上乗せが可能な既存薬の多くは体重増加や低血糖の懸念があるが、SGLT2 の阻害という作用機序はこれらの問題を回避し、また尿糖排泄の促進作用により総カロリーの消費が進むため体重が減少する可能性もある という。一方、本薬剤には軽度の浸透圧利尿がみられるため、頻尿や多尿の懸念があるという。 標準治療に対する非劣性を無作為化試験で検証 CANTATA-SU試験は、メトホルミン単独では血糖値の管理が十分でない2型糖尿病患者において、カナグリフロジンの上乗せ効果の、グリ メピリド(商品名:アマリールほか)に対する非劣性を検証する二重盲検無作為化第III相試験。対象は、年齢18~80歳、HbA1c 7.0~9.5%、 10週以上のメトホルミン投与を受けている2型糖尿病患者であった。 これらの患者が、カナグリフロジン 100mgまたは300mg、あるいはグリメピリド(6から8mgへ漸増)を1日1回経口投与する群に無作為化に 割り付けられた。主要評価項目はベースラインから治療52週までのHbA1cの変化で、非劣性マージンは0.3%とした。 100mg群の非劣性、300mg群の優位性を確認 2009年8月28日~2011年12月21日までに19ヵ国157施設から1,450例が登録され、グリメピリド群に482例、カナグリフロジン 100mg群に 483例、300mg群には485例が割り付けられた。 全体の平均年齢は56.2歳(9.2 SD)、男性52%、白人67%、アジア人20%、平均HbA1c 7.8%、平均空腹時血糖9.2mmol/L (≒165.6mg/dL)、平均体重86.6kg、平均BMI 31.0、平均罹病期間6.6年(中央値5.0年)であった。 治療52週時のHbA1cは3群ともにベースラインよりも低下し、最小二乗平均値の変化率はグリメピリド群が-0.81、カナグリフロジン 100mg 群は-0.82%、300mg群は-0.93%であった。 カナグリフロジン 100mg群とグリメピリド群の最小二乗平均値の差は-0.01%(95%信頼区間[CI]:-0.11~0.09)であり、カナグリフロジン 100 mg群はグリメピリド群に対し非劣性であった。また、300mg群とグリメピリド群の最小二乗平均値の差は-0.12%(-0.22~-0.02)であ り、300mg群のグリメピリド群に対する優位性が示された。 体重は、グリメピリド群がわずかに増加したのに対し、2つのカナグリフロジン群は有意に低下した[最小二乗平均値の変化率:0.7%、- 3.7%、-4.0%、100mg群、300mg群とグリメピリド群の差:-4.4(-4.8~-3.9)、-4.7(-5.2~-4.3)]。 良好な安全性プロフィール、浸透圧利尿関連イベントは多い傾向 重篤な有害事象は、カナグリフロジン 100 mg群が24例(5%)、300mg群が26例(5%)、グリメピリド群は39例(8%)に認められた。 カナグリフロジン群で多い有害事象として性器真菌感染症が挙げられ、女性では100mg群が26例(11%)、300mg群が34例(14%)で、グリ メピリド群は5例(2%)であり、男性では100mg群が17例(7%)、300mg群が20例(8%)で、グリメピリド群は3例(1%)だった。 尿路感染症も100mg群が31例(6%)、300mg群が31例(6%)で、グリメピリド群の22例(5%)より多い傾向がみられた。浸透圧利尿関連イ ベントもカナグリフロジン群で多い傾向にあり、頻尿が100mg群、300mg群ともに12例(3%)ずつ、グリメピリド群は1例(<1%)にみられ、多 尿はカナグリフロジン群が4例(<1%)ずつ、グリメピリド群は2例(<1%)に認められた。 著者は、「これらの結果は、メトホルミンで血糖管理が不十分な2型糖尿病患者において、カナグリフロジンは実行可能な治療選択肢である ことを示すもの」と指摘している。