* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download No Slide Title
Condensed matter physics wikipedia , lookup
Anti-gravity wikipedia , lookup
Woodward effect wikipedia , lookup
Gibbs free energy wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Photon polarization wikipedia , lookup
Electromagnet wikipedia , lookup
Superconductivity wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Time in physics wikipedia , lookup
Nuclear structure wikipedia , lookup
Atomic nucleus wikipedia , lookup
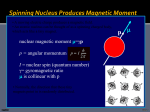
NMR excitation (detecting NMR) • Last time we saw how an ensemble of spins (of a single type and I = 1/2) generates an average magnetization, Mo, upon interaction with an external magnetic field, Bo: z z Mo x y x y Bo Bo • We also saw that this magnetization is proportional to the population difference of spins in the low and high energy levels, and that it is precessing at a frequency wo, the Larmor frequency of the particular observed spin at a particular Bo • So far, nothing happened. We need to do something to the system to observe any kind of signal. What we do is take it away from this condition and observe how it goes back to equilibrium. This means affecting the populations... NMR excitation (continued) • We need the system to absorb energy. The energy source is an oscillating electromagnetic radiation generated by an alternating current: z B1 = C * cos (wot) Mo x B1 Bo y i Transmitter coil (y) • How is that something that has a linear variation can be thought as circular field? A linear variation in y is the linear combination of two counter-rotating circular fields: y y y -wo x = x + +wo x For part of the period of oscillation: = + = + • We go through zero and then it repeats… = + • Only the one vector that rotates at +wo (in the same direction of the precession of Mo) interacts with the bulk magnetization Now we throw Mo on the mix • When the frequency of the alternating current is wo, the frequency of the right vector of B1 is wo and we achieve a resonant condition. The alternating magnetic field and Mo interact, there is a torque generated on Mo, and the system absorbs energy : z Mo B1 wo z x B1 off… x Mxy (or off-resonance) y y wo • Since the system absorbed energy, the equilibrium of the system was altered. We modified the populations of the Na and Nb energy levels. • Again, keep in mind that individual spins flipped up or down (a single quanta), but Mo can have a continuous variation. Return of Mo to equilibrium (and detection) • In the absence of the external B1, Mxy will try to go back to Mo (equilibrium) by restoring the same Na / Nb distributiuon. We’ll see the physics that rule this phenomenon (relaxation) later. • Mxy returns to the z axis precessing on the <xy> plane (to damm hard to draw…): z z x Mxy Mo equilibrium... x wo y y • The oscillation of Mxy generates a fluctuating magnetic field which can be used to generate a current in a coil: z x Mxy wo y Receiver coil (x) NMR signal Laboratory and Rotating frames • The coordinate system that we used for the previous example (laboratory frame) is really pathetic. The whole system is spinning at wo, which makes any kind of analysis impossible. • Again, an out-of-date example. It would be like trying to read the label of a long play spinning in a turn-table… • The solution is to take a coordinate system that moves at wo. This is like jumping on top of the long play to read the label. What we effectively do is remove the effect of Bo. If we take magnetization on the <xy> plane: z z x Bo Mxy y x wo Laboratory Frame Mxy y Rotating Frame • In this coordinate system, Mxy does not move if we are at the resonant condition (the w of B1 is exactly the frequency of the nuclei, wo). If we are slightly off-resonance, the movement of the vectors is still slow with respect to wo. Chemical shifts • If each type of nucleus has its characteristic wo at a certain magnetic field, why is NMR useful? • Depending on the chemical environment we have variations on the magnetic field that the nuclei feels, even for the same type of nuclei. It affects the local magnetic field. Beff = Bo - Bloc --- Beff = Bo( 1 - s ) • s is the magnetic shielding of the nucleus. Factors that affect it include neighboring atoms, aromatic groups, etc., etc. The polarization of the bonds to the observed nuclei are also important. • As a crude example, ethanol looks like this: HO-CH2-CH3 low field high field wo The NMR scale (d, ppm) • We can use the frequency scale as it is. The problem is that since Bloc is a lot smaller than Bo, the range is very small (hundreds of Hz) and the absolute value is very big (MHz). • We use a relative scale, and refer all signals in the spectrum to the signal of a particular compound. d= w - wref wref ppm (parts per million) • The good thing is that since it is a relative scale, the d in a 100 MHz magnet (2.35 T) is the same as that obtained for the same sample in a 600 MHz magnet (14.1 T). • Tetramethyl silane (TMS) is used as reference because it is soluble in most organic solvents, is inert, volatile, and has 12 equivalent 1Hs and 4 equivalent 13Cs: CH 3 H3C Si CH 3 CH 3 • Other references can be used, such as the residual solvent peak, dioxane for 13C, or TSP in aqueous samples for 1H. Scales for different nuclei • For protons, ~ 15 ppm: Acids Aldehydes Alcohols, protons a to ketones Aromatics Amides Olefins Aliphatic ppm 15 10 7 5 2 0 TMS • For carbon, ~ 220 ppm: C=O in ketones Aromatics, conjugated alkenes Olefins Aliphatic CH3, CH2, CH ppm 210 150 C=O of Acids, aldehydes, esters 100 80 50 0 TMS Carbons adjacent to alcohols, ketones Chemical shift in the rotating frame • We will consider only magnetization in the <xy> plane. We start with a signal with an wo equal to the w of B1. After some time passes, nothing changes… y y Time (t) x x • Now, if we are slightly off-resonance (w - wo 0), the Mxy vector will evolve with time. The angle will be proportional to the evolution time and w - wo (that’s why we use radians…) y y Time (t) x f w - wo f = (w - wo) * t x Coupling Constants • The energy levels of a nucleus will be affected by the spin state of nuclei nearby. The two nuclei that show this are said to be coupled to each other. This manifests in particular in cases were we have through bond connectivity: 1 H 13 1 1 H H three-bond C one-bond • Energy diagrams. Each spin now has two energy ‘sub-levels’ depending on the state of the spin it is coupled to: I bb S ab J (Hz) ba S I aa I S • The magnitude of the separation is called coupling constant (J) and has units of Hz. • Coupling patterns are crucial to identify spin systems in a molecule and to the determination of its chemical structure. Couplings in the rotating frame • We will consider an ensemble of spins I coupled to another spin S that is exactly at the resonant condition (w of B1 is wo), and again, only what goes on in the <xy> plane. • The situation is analogous to what happened with chemical shift. In this case, since there are two new energy levels for the spin, we get two counter-rotating vectors. Their evolution will depend on the magnitude of J, not wo: y y -J/2 t ... x x t=1/J +J/2 y t=2/J y x x f=p*t*J Take-home message… a) Under the effect of chemical shifts, components of the total magnetization of a sample will move faster or slower than the reference frequency (i.e. the chemical reference, TMS). b) Under the effect of scalar coupling, an ensemble of spins I will separate in two counter rotating vectors at speeds of + and - J / 2. We have a p involved due to the Hz to radians conversion. c) The time we let the system evolve is extremely important. By varying the evolution time we’ll be able to see different things related to the sample. Next class topics • Instrumentation. • Continuous Wave (CW) excitation. • Pulses and Fourier Transformation. • The free induction decay (FID). • Data collection, processing, window functions, and zero-filling.