* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Organic for Chem II
Survey
Document related concepts
Transcript
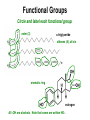
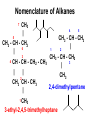
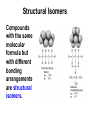
UNIT 6 Theories of Covalent Bonding and Intro to Organic Chemistry Functional Groups and Organic Nomenclature 3-D Structure **You are responsible for being able to draw accurate 3-D structures of relatively simple organic compounds!** You must correctly show all bond angles (this includes not “splitting” bonds) which atoms are in the plane of the paper which atoms are in front of the plane of the paper which atoms are behind the plane of the paper Start practicing now Carbon Carbon has an exceptional ability to bond with itself, forming a variety of molecules with chains or rings of C atoms. Carbon also forms strong bonds with H, O, N, and the halogens. Consequently, C can form millions of compounds. Carbon Carbon has 4 valence electrons and forms 4 bonds. These 4 bonds can be a combination of single, double, and triple bonds. methane, CH4 formaldehyde, CH2O acetonitrile, CH3CN Carbon Bond lengths: C—C > C=C > C≡C Bond strengths: C≡C > C=C > C—C Carbon Hydrocarbons containing no multiple bonds are not very reactive. Organic compounds get their reactivity and their characteristics from functional groups such as -OH (alcohol) and -COOH (acid). Recognizing functional groups is a “must.” Alkanes and Cycloalkanes Have no Functional Groups Compounds that contain only C and H and only single bonds are called either alkanes or, if the C’s form rings, cycloalkanes. They have no functional groups. cyclohexane, a cycloalkane hexane, a straight chain alkane formula C6H14 formula C6H12 There Are Many Ways to Show Organic Structures • The structures shown on the previous slide are called condensed structures or condensed structural formulas. • Lewis structures show all bonds and all nonbonding valence electrons. Lewis structures condensed structural formulas Line Angle Structures Organic compounds can be really large. Imagine how many C’s and H’s and their bonds you’d have to show: the structure would be very “busy.” Lewis structures line angle structures Line Angle Structures Line angle structures are another way to represent organic molecules, a way that lets you focus on functional groups. No C’s are shown, and almost no H’s bonded to C’s are shown, either. Lewis structures line angle structures* *There is a C atom at either end of every line segment. The H’s on each C are enough to give the C four bonds total. YOU MUST BE ABLE TO COUNT TO 4. Functional Groups - Alkenes • Clearly, line angle structures are less “busy.” • C=C is the alkene functional group, also called the carbon-carbon double bond. Lewis structure line angle structure trans-2-hexene (The double bond starts on carbon number 2. One counts from the end that gets to the C=C bond first.) cyclohexene Which Carbon has the Functional Group? • Organic compounds are “read” and named from the end that brings you to the functional group first. All structures shown below are for the same compound, trans-2-hexene. Lewis structures line angle structures condensed structural formulas Functional Groups - Alkynes C≡C is the alkyne functional group, also known as a carbon-carbon triple bond. Lewis structure line angle structure 2-hexyne (The triple bond starts on C number 2. One counts from the end that gets to the carbon-carbon triple bond first.) Functional Groups Alkyl Halides Alkyl halides (-X) all have a carbon atom bonded to a halogen atom (F, Cl, Br, I). Halogen atoms are designated X. 2-bromopropane or propyl bromide, CH3CHBrCH3 Lewis structure line angle structure Functional Groups Aromatic rings • Several structures are aromatic, but we will look at only one: the benzene ring, also called an aromatic ring. • Benzene is NOT three alkenes! It is a unique structure where six electrons are shared among all 6 carbon atoms. That is why the line angle structure is often seen with a circle in the middle of the ring. Lewis structure line angle structures benzene, C6H6 Aromatic Hydrocarbons contain 6membered ring structures with alternating double bonds, stabilized by the delocalized electrons from the pi bonds. are not as reactive as the alkenes and alkynes. Functional Groups alkene -X halide aromatic ring alkyne Functional Groups - Alcohols Alcohols all have the –OH group (called a hydroxyl group and sometimes shown as HO-). Isopropyl alcohol or 2-propanol, CH3CHOHCH3 Lewis structure line angle structure Ethers • are like water, with alkyl groups replacing both of the -Hs: H-O-H R-O-H R-O-R’ water alcohol ether • are relatively unreactive. • are commonly used as solvents. Functional Groups - Amines Amines are organic bases and have the formula RNH2, RNR’R”, or RNHR’ , where R is an alkyl group. CH3CH2NH2 ethylamine (CH3)3N trimethylamine pyrrolidine All three functional groups in blue are amines. Functional Groups alcohol ether R = alkyl group, which is a group containing C’s and H’s with no multiple bonds. amine (1°) Some Aldehydes and Ketones O ║ R-C-R' O ║ R-CH O ║ HCH methanal (formaldehyde) C=O is called a carbonyl group ketone aldehyde O O ║ ║ CH3CH CH3-CH2-CH ethanal (acetaldehyde) propanal O ║ CH3-C-CH3 propanone (acetone) Functional Groups Carboxylic Acids Carboxylic acids (-COOH) are organic acids. lauric acid, CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2COOH Lewis structure line angle structure Note that the -OH group and the =O are on the same C atom. Functional Groups - Esters The fats in our bodies are esters. Below is a simple ester. ethyl acetate, CH3CO2CH2CH3 Lewis structure line angle structure The ester group is in green. Although carbon and most hydrogen atoms aren’t shown, other atoms are. Functional Groups Amines, Amides, and Nitriles amines amides Amides are like amines, but with an adjacent C=O. Be careful! Both amines and amides have different forms: primary (1°), secondary (2°), and tertiary (3°). Nitriles are R-CN. Functional Groups aldehyde carboxylic acid amide - C≡N nitrile ketone ester Functional Groups Circle and label each functional group. alcohol alkenes (5) carboxyl group vitamin A (retinol) ester aromatic ring…NOT three alkenes! aspirin Functional Groups Circle and label each functional group. ester (3) a triglyceride alkenes (4), all cis aromatic ring estrogen All -OH are alcohols. Note that some are written HO-. 3-D Structure Solid lines are bonds in the plane of the board or paper. Wedges show bonds to atoms in front of the plane. Dashed lines show bonds to atoms behind the plane. 3-D Structure **You are responsible for being able to draw accurate 3-D structures of relatively simple organic compounds!** You must correctly show all bond angles (this includes not “splitting” bonds) which atoms are in the plane of the paper which atoms are in front of the plane of the paper which atoms are behind the plane of the paper Start practicing now 3-Drawing Structure Now, draw the 3-D structure for this compound. CH3 CH2 CH2CH2COOH Nomenclature Organic compounds get their reactivity and their characteristics from functional groups. Organic compounds are named after the functional groups they contain. We will start with the alkanes. Alkanes are characterized by stable C-C single bonds are aka saturated hydrocarbons. are named for the number of carbons in the longest chain. have no functional groups. Alkanes Molecular formula CH4 C2H6 C3H8 C4H10 C5H12 C6H14 C7H16 C8H18 C9H20 C10H22 Condensed Structural Formula CH4 CH3CH3 CH3CH2CH3 CH3CH2CH2CH3 CH3CH2CH2CH2CH3 CH3CH2CH2CH2CH2CH3 CH3CH2CH2CH2CH2CH2CH3 CH3CH2CH2CH2CH2CH2CH2CH3 CH3CH2CH2CH2CH2CH2CH2CH2CH3 CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 Name methane ethane propane butane pentane hexane heptane octane nonane decane Nomenclature • Systematic naming comes from the International Union of Pure and Applied Chemistry (IUPAC). • Below is the template you will use to build the name of ANY organic compound. stereomain functional substituents unsaturation isomerism chain group Nomenclature of Alkanes 1. Find the longest continuous chain of C atoms, and use that as the base name of the compound. 2. If there is more than one long chain, choose the one that gives more substituents. 3. Number the C atoms in the longest chain, beginning with the end that brings you to the substituent sooner. 4 1 5 CH3 - CH - CH3 | 2 CH3 - CH - CH2 3 | CH3 Nomenclature of Alkanes 3. Name and give the location of each substituent group. Condensed Structural Formula Name CH3— CH3CH2 — CH3CH2CH2 — CH3CH2CH2CH2 — CH3CH2CH2CH2CH2 — (CH3)2CH — (CH3)3C— methyl ethyl propyl butyl pentyl isopropyl t-butyl Nomenclature of Alkanes 4. When two or more substituents are present, list them in alphabetical order. Condensed Structural Formula Name CH3— CH3CH2 — CH3CH2CH2 — CH3CH2CH2CH2 — CH3CH2CH2CH2CH2 — (CH3)2CH — (CH3)3C— methyl ethyl propyl butyl pentyl isopropyl t-butyl Nomenclature of Alkanes CH3 | 7 5 CH3 - CH - CH2 6 | 3 4 CH - CH - CH2 - CH3 | 4 1 CH3 2 2,4-dimethylpentane CH3 3-ethyl-2,4,5-trimethylheptane 1 CH3 - CH - CH3 | 2 CH3 - CH - CH2 3 | | CH3 CH - CH3 | 5 Drawing an Alkane from its Name Draw the structure of 3-ethyl-2-methylpentane. 1. Write the backbone (pentane). 2. Put in each substituent at the correct C and adjust the hydrogens to keep 4 bonds per C. CH2 - CH3 | CH3 - CH - CH - CH2 - CH3 | CH3 Cycloalkanes Hydrocarbons where some of the C atoms form rings. Reactive because of the strain caused by the 60° bond angle. Structural Isomers Compounds with the same molecular formula but with different bonding arrangements are structural isomers. Structural Isomers of Pentane 3-D Structures Draw the 3-D structures of : pentane acetaldehyde, CH3CHO Geometry of Alkanes Rotation about a C-C single bond is relatively easy, and it occurs very rapidly at room temperature. Although we talk about straight-chain hydrocarbons, the alkanes constantly undergo motions that cause them to change their shape, like a chain being shaken.