* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download BATTERIES
Survey
Document related concepts
Transcript
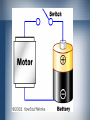
BATTERIES TEAM: ATE Names of Team Members Goals & Purpose • • • • • Redox Reactions Explain how batteries work Explain how energy is produced Explain Battery Arrangements Different types of batteries Batteries are EVERYWHERE! • • • Technology in US continuously advancing-smaller batteries w/ more power flashlights, pagers, cell phones, ipod, laptops, cars, etc. dependent on batteries and how long they keep their charge REDOX REACTIONS • AKA Oxidation-Reduction Reactions • transfer of electrons between atoms • Oxidation involves a loss of electrons • Reduction involves a gain in electrons BATTERIES • • • • Put redox reactions to use Convenient way of generating electricity Electrical energy can be obtained from any electrochemical cell When several cells are connected to provide sufficient power- these cells are known as BATTERIES How batteries work… • • • • • Every battery has two terminals – Positive + & Negative Normal flashlight batteries the ends are the terminals Large car batteries have two heavy lead posts that act as terminals Electrons collect on negative end Connect wire between two terminals & electrons flow through it from the negative to the positive terminal • • • Electrons must flow from battery through wire from the negative to the positive terminal for the chemical reaction to take place The speed of electron production by this chemical reaction controls how many electrons can flow between the terminals Unless electrons are flowing reaction does not take place… this is why batteries can sit for years and still work Battery Arrangements • Group together serially to form higher voltage (voltages add up) • Group together parallel to form higher current (currents add up) CURRENT – flow of electric charge • Electronic and electrical things 'work' because electrons are flowing through them • This is current (I) measured in amps. • The more amps, the hotter, faster, louder, etc • Talking of current is always relative to a SINGLE POINT in a circuit... the number of electrons passing that point VOLTAGE – potential energy • • • What makes the electrons move Voltage is always a comparison between two points one point is assumed to be the 'zero voltage' point Types of Batteries • Zinc-Carbon- standard carbon battery used • Alkaline- Common in Duracell & Energizer • Lithium Photo- lithium, lithium iodide, and • Lead-acid-automobiles – oxide electrodes – • Nickel-cadmium- nickel-hydroxide & in all inexpensive AA, C, & D dry cell batteries batteries – zinc & manganese-oxide lead-iodide used – ability to supply power surges (rechargeable) potassium-hydroxide electrodes (rechargeable) Types continued… • Nickel-metal hydride- rapidly replacing nickel due to not suffering from memory effect (rechargeable) • Lithium-ion-very good power-to-weight ratio – high-end laptop computers and cell phones (rechargeable) • • • Zinc-air- lightweight and rechargeable Zinc-mercury oxide- hearing aids Silver-zinc- aeronautical applications – good power-to-weight ratio • Metal-chloride- electric vehicles - scooter QUIZ Question… • Name seven things that you personally use batteries for: SOURCES • • • • • Baird, Colin and Gloffke, Wendy. (2005). Chemistry in Your Life. New York: W. H. Freeman and Company. http://www.howstuffworks.com/ http://www.chemistry.mnsu.edu/ http://www.duracell.com/landing.asp http://www.energizer.com/