* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 13.1 Electrochemical Cells
Survey
Document related concepts
Transcript
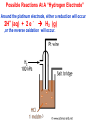
Electrochemical Cells Electrochemical and Electrolytic Cells An electrochemical cell uses chemical reactions to produce electrical current, to release potential electrical energy. An electrolytic cell uses electrical energy to produce desired chemical reactions at electrodes like electroplating an electrode. Electrochemical Cell Electrolytic Cell Electrochemical and Electrolytic Cells The anode in an electrochemical cell (galvanic cell) is a negative electrode or terminal while the cathode is a positive terminal. The anode in an electrolytic cell is a positive terminal while the cathode is a negative terminal. In both of these cells, the terminal where oxidation occurs is the anode while the terminal where reduction occurs is the cathode. This can be remembered with the mneumonic, “An Ox CaRed”. Electrochemical Cell Electrolytic Cell Parts of an Electrochemical Cell • The anode is the electrode where oxidation occurs, where electrons flow away from, where solution anions move towards. • The cathode is the electrode where reduction occurs, where electrons move towards, where solution cations move towards. oxidation reduction Remembering Electrode Reactions The memory aid, “an ox cared” , stands for anode-oxidation, cathode-reduction. This relationship holds true for both electrochemical cells and electrolytic cells. Features of an Electrochemical Cell 1. Referring to a standard reduction potentials table, it can be seen that in comparing the copper and zinc electrodes, copper has a greater tendency to reduce while zinc has a greater tendency to oxidize. Features of an Electrochemical Cell 2. As Zn oxidizes, the Zn+2 ions migrate into solution leaving electrons behind in the Zn electrode (making it negative). The Zn electrode is the anode (An ox cared). The anode loses mass. 3. Copper ions Cu+2 , from the solution next to the copper electrode, reduce at the copper electrode which plate new copper metal onto the copper electrode. The Cu electrode is the cathode. The cathode gains mass. Features of an Electrochemical Cell 4. Electrons flow from the anode (Zn) to the cathode (Cu), from – to +. 5. Without the salt bridge, Zn+2 ions would build up around the Zn electrode pulling onelectrons, keeping them from flowing away. In addition (without the salt bridge), Cu+2 ions from solution plating on the Cu electrode leave the solution overall negative (SO4-2 anions from the CuSO4 compound). These anions repel incoming electrons, stopping the flow. Features of an Electrochemical Cell 6. The function of the salt bridge is to neutralize the charge build-up around each electrode due to solution ions. Positive ions from the salt bridge move to the cathode to neutralize the minus charge of the solution anion build-up. Negative ions from the salt bridge move to the anode to neutralize the positive charge build-up due to Zn+2 cations released into solution by the Zn anode. Features of an Electrochemical Cell 7. Electrons only flow through the external wire (not in the solutions) while solution ions migrate in the solution. 8. The ideal voltage that can be produced at the start of the cell’s operation is found by adding the voltages of the half cell reactions. Note that since Zn is oxidized, the reaction below must be written in reverse with the sign changed from -0.76 to +0.76. Predict Anode, Cathode, Current Direction, Mass Changes and Initial Voltage KNO3 solution Al Al(NO3)3 solution Ag AgNO3 solution What Is Voltage? Voltage is a measure of the potential energy carried by flowing electrons. It is the energy per coulomb (6.24 x 1018) of electrons. 1 V is 1J/C. Potential Difference Electrons do not flow in a single half-cell so an individual half-cell voltage cannot be determined. However, if two half cells are connected, the potential difference between those two cells can be measured. Iron Half-Cell Fe (s) 1M Fe(NO3)3 solution A Review of Electrical Terminology A coulomb (C) is a number of electrons (1C = 6.24 x 1024 e-). Electrical current (amperes – A) is the number of electrons passing per second, A = #C/s. Voltage is the energy carried per number of electrons, V = J/C. Standard Reduction Potentials All half cell reactions are assigned voltages in terms of being connected to a hydrogen half-cell. A hydrogen half-cell has hydrogen gas at 1 atm (101.3 kPa) pressure bubbling into a glass tube which has an inert (non-reactive) platinum (Pt) electrode. There may be a return tube carrying excess H2 (g) away. The electrode is suspended in a 1 M solution of strong acid so that there is 1 M H+ in solution. Possible Reactions At A “Hydrogen Electrode” Around the platinum electrode, either a reduction will occur 2H+ (aq) + 2 e - H2 (g) ,or the reverse oxidation will occur. Standard Reduction Potentials Arbitrarily, the hydrogen half cell is assigned a value of zero and the other cell’s voltage is determined experimentally with a voltmeter. The hydrogen half-cell reaction is : 2H+ (aq) + 2 e - H2 (g) ; Eo = 0.000 V Experimentally Determining a Standard Reduction Potential Under standard conditions, a second electrode is attached to a hydrogen electrode with a voltmeter between. The reading on the voltmeter establishes where on the chart the half reaction will be placed. Note the placement of the Ag+ reduction half reaction in the SRP chart. 2Ag+ + H2 2H+ + 2Ag ; Eo = 0.80 V Experimentally Determining a Standard Reduction Potential In the new reaction shown, H+ is being reduced while Zn is being oxidized. A voltage of +0.76 V is produced when Zn is oxidized. To write a reduction potential for Zn2+, the voltage must be reversed since the half reaction is reversed. Note the placement of Zn2+ in the SRP chart. 2H+ + Zn H2 + Zn2+ H2 + Zn2+ 2H+ + Zn ; Eo = 0.76 V ; Eo = -0.76 V The Meaning of Eo Eo stands for Standard Reduction Potential where the “ o “ means standard state. The standard state for a half-cell is 25o C, all gases in the cell at 101.3 kPa of pressure, all elements in the cell in their standard states at 25o C, all solutions in the cell at a concentration of 1 M. The Sign of Eo When a half-reaction is reversed from how it is written in a table (when an oxidation is occuring), the sign of the Eo is reversed. The Eo values of the reduction and oxidation reactions are added to give the voltage value of an electrochemical cell as it starts under standard conditions. What The Eo Sign Means A positive E sign means that the cell will do work (produces electricity – the reactions are spontaneous) A negative E sign means that the cell has to be given energy for the reactions to occur – the reactions are not spontaneous. Al+3 + 3Ag Al + 3Ag+1 is ? Zn+2 + Mg Zn + Mg+2 is ? Balancing Coefficients Have No Effect on Eo For the reaction, 3 Ag+ + Al 3 Ag + Al+3, The half reactions are: 3 Ag+ + 3 e- 3 Ag ; Eo = +0.80 V Al Al+3 + 3 e- ; Eo = +1.66 V Eocell = + 2.46 V Note that the Eo value is unaffected by the coefficient, 3. The reason for this is that if there are 3 x the electrons capable of doing 3X the work, the voltage ratio of work/charge remains the same. 3X work / 3X electrons = work / electrons Area of the Electrodes has No Effect on Eo The potential difference Eo does not change when the area of the electrodes is changed. The reaction rate does increase when the surface area increases and this releases more electrons per second which increases the amperage or current flow. A larger electrode surface area also increases the time that a cell can operate. The Size of Eo and Rate of Reaction In the reaction: 3 Ag+ + 3 e- 3 Ag ; Eo = +0.80 V Al Al+3 + 3 e- ; Eo = +1.66 V Eocell = + 2.46 V The size of Eo does NOT indicate anything about the rate of reaction. This reaction normally proceeds very very slowly. This is because aluminum reacts with oxygen in a solution to form an oxide coating (Al2O3) on the aluminum metal that adheres strongly and inhibits further reaction unless sufficient activation energy is supplied. Aluminum oxide Sil ver Aluminum Detarnishing Silverware Tarnished silverware is Ag2O which when reacted with Al causes silver (Ag+) to be reduced to Ag : 3 Ag+ + 3 e- 3 Ag ; Eo = +0.80 V Al Al+3 + 3 e- ; Eo = +1.66 V Eocell = + 2.46 V This reaction is accomplished by heating tarnished silverware on aluminum foil with baking soda (makes a weak base that removes the aluminum oxide coat) . E Values for Nonstandard Conditions Increasing concentrations of solutions causes a voltage rise since an increase in reactant concentration tends to shift equilibrium to the products. 2 Ag+ + 2e- 2Ag ; ½ react. Eo = +0.80 Cu2+ + 2e- Cu ; ½ react. Eo = -0.34 , Cell Eo = +0.46 V 2 Ag+ + 2e- Cu2+ + 2e- 2Ag Cu E > +0.80 E > -0.34 A reduction in reactant concentration causes a shift towards the reactants ( a greater tendency for the reverse reaction) which causes a voltage drop from the expected Eo. 2 Ag+ + 2e- 2Ag Eo < +0.80 Cu2+ + 2e- Cu Eo < -0.34 When Cells Reach Equilibrium When a cell starts, it produces the full theoretical Eo voltage value. As the cell operates, this voltage gets lower and lower. The reason for this is that as products build up, the opposing reactions have a greater tendency. Ex: 2 Ag+ + 2e- 2Ag Cu2+ + 2e- Cu Thus the starting Eo drops for the reduction reaction and also drops for the oxidation reaction so that the final E is 0.00 V Selecting Preferred Reactions In cells where multiple reduction or multiple oxidations are possible, the reduction and oxidation reaction with the highest potential (E) will be the ones that occur. Example 1 of Selecting the Preferred Reaction Example 2 of Selecting the Preferred Reaction The Lead-Acid Storage Battery A charged car battery (Lead-Acid) has electrodes of Pb and PbO2 . It can produce electric current with the electrodes getting a lead IV sulfate coating and the reactions can be reversed by supplying electric current to produce the original Pb and PbO2 electrodes. The Reactions in a Lead-Acid Storage Battery Typical Cell Construction of a 12 V Car Battery Car batteries typically are made up of six 2 volt cells connected in series to yield 12 V. Different Acid Concentration When Discharged As a lead-acid storage battery discharges, sulfuric acid is used up and water is produced. This reduces the solution’s acid concentration and its density becomes less (from 1.30 to 1.10 g/mL). A floating hydrometer will float lower in a discharged battery. Determining Which Electrode is the Anode/Cathode The anode is the electrode where oxidation is occurring while the cathode is where reduction is occurring. The menumonic, “An Ox CaRed” can be used to remember this. The anode in an electrochemical cell is negative while the cathode is positive. The Zinc-Carbon Battery Fuel Cells The electron flow shown in this diagram is wrong and should be in the opposite direction. Ballard Hydrogen Oxygen Fuel Cell Current (electrons) flows from the anode (-), where hydrogen gas is releasing electrons (oxidized) to become positive protons, to the cathode (+) where oxygen is accepting electrons (from the current) and protons to make water. Corrosion of Iron (Called Rusting) Deep in the centre of a water drop, which is oxygen-poor, iron metal oxidizes to ions, releasing electrons (Fe Fe2+ + 2e-). The electrons migrate to the outer edges where they reduce dissolved oxygen (in the oxygen-rich outer region of the drop) to hydroxide ions (1/2 O2 + H2O +2e - 2OH-). The 2OH- ions react with the Fe2+ to produce insoluble Fe(OH)2 at the outer edges of the water drop. Fe(OH)2 is then oxidized to Fe2O3 . XH2O (rust) due to further contact with oxygen. ½ O2 + H2O + 2e- 2OH- Fe2+ + 2OH- Fe(OH)2 Fe Fe2+ + 2e- Preventing Corrosion Generally, three things can be used to prevent metals from corroding. 1. Use a metal whose oxide layer adheres strongly to the metal. The oxide coating then prevents water and oxygen from making contact with the metal. Aluminum and copper produce oxides that bind to the metal and protect it from further oxidation. Preventing Corrosion 2. Another method of preventing corrosion is to apply a protective coating or a paint coating to a metal which then prevents oxygen and water from reacting with the metal. Preventing Corrosion 3. Another method of preventing corrosion is to plate a second metal onto a metal that tends to corrode. The second, plated metal tends to oxidize easier but has an oxide that strongly adheres to the second metal forming a barrier to oxygen and water. Steel can be plated with tin whose oxide (SnO2) forms a protective layer. Galvanized nails are iron coated with zinc whose oxide (ZnO) also strongly adheres as a protective layer. Cathodic Protection Cathodic protection means that a metal to be protected against corrosion (like iron) is connected to another metal (like magnesium) that has a greater tendency to oxidize. Since the other metal (magnesium) is oxidized before the protected metal (iron) it is connected to, the protected metal receives electrons (acting as a cathode) from the metal giving electrons (the anode) [An Ox Cared]. In essence the magnesium is sacrificed to prevent iron from oxidizing. Cathodic Protection Some pipes, ships’ hulls and vehicles are cathodic protected by putting a low voltage (low energy) current into them, making the metal unable to give off electrons (corrode). The current provides electrons to surrounding water and oxygen rather than the metal. Cathodic Protection Sacrificial anodes and impressed electrical current is commonly used to protect underground metal storage tanks. Changing Chemical Surroundings to Fight Corrosion The reduction reaction of oxygen causes the oxidation of metals like iron. ½ O2 + 2H+ (10-7 M) + 2e- H2O ; E= 0.82 V If oxygen can be removed from a solution in contact with a metal, the only reduction that can occur higher than iron is 2 H+ (10 -7 M) + 2e- H2 (g) ; E = -0.45 V which is a much slower reaction. Even better protection is obtained by adding OH- ions (NaOH) to a solution around the metal. The reduction half reaction is now 2 H2O + 2e- H2 (g) + 2 OH- ; E = -0.83 V which is below iron in the SRP chart. End of Presentation

























































![Electricity and Magnetism [2]](http://s1.studyres.com/store/data/007809428_1-2a02c27dae15966177eeec747c9ebd14-150x150.png)