* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Health Physics Course 16.739 Instrumentation I
Mercury-arc valve wikipedia , lookup
Vacuum tube wikipedia , lookup
Resistive opto-isolator wikipedia , lookup
Buck converter wikipedia , lookup
Switched-mode power supply wikipedia , lookup
Stray voltage wikipedia , lookup
Cavity magnetron wikipedia , lookup
Alternating current wikipedia , lookup
Opto-isolator wikipedia , lookup
Mains electricity wikipedia , lookup
Voltage optimisation wikipedia , lookup
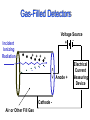
Instrumentation and Detection Harry M. Johnson, PhD CancerCare Winnipeg, Manitoba, Canada Instrumentation and Detection • Purpose: To discuss the detection of radiation from the point of view of the classical instrumentation. Instrumentation and Detection: Outline • • • • • • • • The Basic Gas-Filled Detector Classes of Gas-Filled Detectors The Geiger Tube as an Exposure Meter Choosing a Radiation meter Scintillation Detectors Calibrating a Contamination Meter Neutron Meter Thermoluminescent Personnel Dosimetry The Gas-Filled Detector - 1 • Gas-filled counting chamber • Use air as the gas for initial testing • sealed or unsealed • coaxial electrodes, well insulated • variable voltage, defined anode and cathode • high resistance resistor • capacitance C • radiation enters chamber and ionizes the gas • ions drift towards the electrodes • Voltage pulse is detected The Gas-Filled Detector - 2 • Apply low voltage • a few ion pairs drift to electrodes • time constant RC is long • Voltage V= Q/C is detected at output • Pulse curve has elongated shape • Difficult to detect successive pulses The Gas-Filled Detector - 3 • Maintain the same voltage • “drifting” of ion pairs drift to electrodes is the mechanism of charge transport from ion pair production • shorten the time constant RC • Voltage V= Q/C continues to be detected at output • Pulse curve has “clipped” shape • Successive pulses are detectable • Now possible to count the individual pulse • This is the preferred chamber design • Possible to calculate maximum pulse rate Gas-Filled Detectors Incident Ionizing Radiation Voltage Source + + + + + - - - Anode + Cathode Air or Other Fill Gas Electrical Current Measuring Device Ion Chamber Instrument • Example of Ion Chamber • 6 cc Chamber • 180 cc Chamber • Readout unit is located remote from the detector The Gas-Filled Detector • The first plateau region is the “ion chamber” mode • Typically 300 volts applied voltage • Pulse size is independent of LET • No secondary ionizations - which would amplify the pulse height • Pulse size distinction is a disadvantage The Ionization Chamber • • • • • • • • • Radiation of constant flux is applied to chamber Vary the voltage in the few-hundreds of volts region ionizations in the air of the chamber create ion pairs a plateau region is noted - the operating plateau for the ion chamber V is just high enough to collect all ions from ionizations Ions are not being accelerated - still “drifting” to electrodes Pulses are distinguishable - each pulse is a single ionization event Pules size is independent of voltage a beta particle produces 1000 ion pairs, output pulse voltage is low millivolts Low output voltage is disadvantageous The Proportional Counter • • • • • • • • • • Adjust the voltage upward beyond the ion chamber plateau Ions from the initial ion pairs are accelerated electron acceleration is more important than positive ions Secondary electrons are produced by collisions by the primary ion-pair products Change the gas in the chamber These new design features create a gas amplification factor (>1) - called an “avalanche” By increasing the tube voltage the avalanche spreads along the anode wire Pulse size is proportional to the chamber voltage Pulse size depends on the electric field gradient Anode (central) diameter is important The Proportional Counter • This is the “Proportional Counter” Region • Note slight upward slope of Voltage-Pulse curve in this region • Pulse size is dependent on LET - giving the ability to discriminate between type of radiation • Anode wire size is typically 0.02 to 0.1 mm diameter • Decreasing the gas pressure increases the multiplication, although the tube may be at atmospheric pressure • High-voltage supply must be very stable The Geiger Tube - 1 • Basic Readout module • Two Geiger tubes: “Pancake” type and the “End-Window” type. • Entrance windows are very thin The Geiger Tube - 2 • Continue to raise the voltage past the “proportional counter” plateau to a new plateau. • Slight upward slope of Voltage-Pulse curve in this region. • Counting rate is independent of voltage. • Change the gas to Argon ( = 15.7 eV; W = 26.4 eV) or Methane ( = 15.2 eV; W = 27.0 eV). • Add 10% ethyl alcohol to eliminate uv . • Tube is sealed. • Chamber pressure may be 10% of atmospheric pressure • Output pulse (without amplification) height:approx 1 volt • Little need for amplification • Output pulses occur independent of size of initiating ionization - no discrimination re LET of the radiation. The Geiger Tube - 3 • This is Geiger (Geiger-Mueller) tube region (1908). • Voltage is sufficiently high that both ions of the initiating ion pair are accelerated. • Accelerated ions cause additional ionizations (avalanche). • Accelerate +ve ions strike cathode (tube shell), cause excitations of cathode molecules, yielding UV production. • UV is additional source of gas ionization/excitation. • When intense ionization occurs in the tube the E-Field along the anode wire drops to zero. This causes dead time. • GM tube can go into continuous discharge when this occurs. Add alcohol to argon gas to quench the discharge. • Alcohol reduces dead time to 100 micro seconds. (Resolve up to 10,000 pulses/sec). • GM tube is easy to build, simple electronics, cheap. The Geiger Tube - 4 • Choose operating voltage for G-M region at 1/3 to 1/2 way up the plateau. • Alcohol is present to quench the ionizations and absorb the UV produced when accelerated +ve ions strike shell. • Alcohol molecules dissociate in this process. • Lifetime of the tube is limited by the alcohol - total lifetime is 108 - 109 ionizations. • Ifd voltage is raised above the Geiger region, the avalanche spreads and continuous discharge occurs. Gas tube cannot operate as a detector above the Geiger region. Resolving Time and Dead Time • Two ionizations in G-M tube in rapid succession may not be resolved. • The first ionization causes a Dead Time when no new pulse can be detected • Followed by Recovery Time when a new pulse may not be identifiable • Resolving Time is sum of Dead and Recovery times Resolving Time and Dead Time - 2 • Avalanche starts near the anode wire, spreads along anode due to the High Voltage in G-M region • Electrons move more quickly than +ve ions • Rate-limiting step is the transit time of +ve ions to the cathode. This transit time defines the collection time • Resolving time can be defined • Tube resets itself after recovery • Collection time may be a few hundred microseconds • If CT is 250 s, what is limiting rate of detectable ionizations - how many photos per sec is max input? Advantages/Disadvantages of Gas Detection Tubes • Ion Chamber: simple, accurate, wide range, sensitivity is function of chamber size, no dead time • Proportional Counter: discriminate hi/lo LET, higher sensitivity than ion chamber • GM Tube: cheap, little/no amplification, thin window for low energy; limited life Geiger Tube as Exposure Meter • “Exposure” is the parameter measuring the ionization of air. • Geiger tube measures ionization pulses per second - a “count rate”. • The number of ionizations in the Geiger tube is a constant for a particular energy but is energy dependent. Energy Compensation of Geiger Tube Energy Response for Victoreen 489-4 GM Probe 10 Ratio Output to Actual Exposure Rate • An energy compensating shield is required to smooth out the energy response Bare Tube Response Response - Beta Shield Closed 1 10 100 1000 0.1 Photon Energy in keV 10000 Scintillation Detector • Readout Module plus Detector • Photomultiplier Tube (P) • Scintillator Chrystal (C) P C Scintillation Detectors Incident Ionizing Radiation Light Photon Photomultiplier Tube Pulse Measuring Device - Sodium-Iodide Crystal Dynode Photocathode Optical Window Anode Scintillation Detectors • Construction of Crystal and PMT assembly • Design of basic electronics • Principle of scintillation • Principle of operation Scintillation Detection System • Design of Basic Pulse Height Analysis System • Amplitudes of voltage pulses are sorted by PHA • PHA counts number of pulses for various voltages (energies) • Display is a histogram of pulse heights Scintillation Detector Materials - 1 Phosphor Rel Pulse Height Decay Time nanosec NaI (Tl) 210 250 Cs I 55 1,100 Plastic 28-48 3-5 ‘Liquid” 27-49 2-8 Scintillators - 2 (Derenzo et al 1992) Phosphor Na I (Tl) Pulse Height photon/MeV 38,000 Decay Time nanosec 230 NE 102A 10,000 2.4 ZnWO4 10,000 5,000 CsI (pure) 59,000 800 CsI (Tl) 2,300 16 CdS(Te) 190 18 Analysis of Scintillator Peak - 1 • NaI (Tl) scintillation peak for Cs-37: 662 keV • Large crystal: 10x10 cm • Only photons that lose all energy (i.e. Compton events + final photoelectric event) contribute to the “Total Energy Peak” Analysis of Scintillation Peak -2 • “Continuous Compton Distribution” arises from light from Compton events and Secondary photons escape from the crystal before the photoelectric event occurs. • “Compton Edge” (478 keV) is the energy of the maximum recoil electron for h = 662 keV. • Tmax = 2 h /(2 + mc2/ h ) = 478 keV; This occurs when the Compton scattering angle (for the secondary Compton photon) is 180 degrees. Analysis of Scintillation Peak -3 • “Backscattered” photon has energy 662-478 = 184 keV. The backscattered peak is visible in the spectrum. • Relative area under the Total Energy Peak (photopeak) depends on the size of the crystal. • Ratio of areas under the “Total Energy” peak and the “Compton Distribution” in a small detector is approximately equal to ratio of photoelectric to Compton cross-section in the crystal material. Analysis of Scintillation Peak -4 • Escape Peak: when the detector is small, the Escape Peak may be visible. • • This peak arises when a K-shell vacancy occurs in iodine (of the NaI material) following a photoelectric event. A characteristic 28 keV x-ray is emitted. Alpha Detectors • Proportional Detectors: counting with discrimination from beta-gamma ionizations. • Scintillation Detector - Zinc Sulphide with discrimination against beta-gamma ionizations by pulse height control and by thin detector efficiency. ZnS Alpha Detector • This alpha detector uses a thin scintillator of zinc sulphide on thin plastic, aluminized to keep out light. • The detector is connected to a rate meter with pulse height discriminator. It senses only alpha radiation & rejects beta and gamma. Neutron Detectors - Choices • The dose equivalent detector: a “rem meter” • Activation foils: cadmium • Bubble detectors Neutron Rem Meter • A gas detection tube (BF3) is located at the centre of a polyethylene sphere with a thin cadmium filter. • Sphere moderates neutrons to permit detection by BF3 tube • Energy range 0.025 eV to 10 MeV • Gamma radiation is rejected TLD Personnel Dosimetry • Themoluminescent crystals of LiF are well suited to personnel dosimetry. • Ionizing radiation creates electron dislocation that remains until heated. • Light output on heating is proportional to dose. Choosing a Meter • • • • • • • Contamination or Radiation? X-ray, Gamma, Alpha or Neutron? Energy Dependence? Response Time: Fast or Slow? Sensitivity: Low doses or high doses? Fixed or Portable? Calibration? Summary • • • • • • • • Beta- Gamma Gas detectors Contamination or Radiation Scintillation Detectors Analysis of PHA histogram of energy spectrum Alpha Detectors Neutron detectors Personal Dosimetry Methods Choosing a Meter