* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ENVI 21 Life in the Ocean
Survey
Document related concepts
Transcript
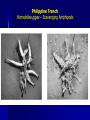
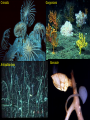
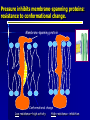
Why should we bother to study deep-sea biology? “..we know more about the moon’s behind than the ocean’s bottom…” Dr. Cindy Lee Van Dover New Yorker classic Most of “biology” (~80%) takes place in the deep sea: The deep sea is the most common habitat in the biosphere! Average depth = 3,800 m I. Deep Sea • A. Life strongly influenced by environmental conditions Conditions 1. Temperature • • 2. Cold – Typically -1 to 4 oC Exceptions • Deep Mediterranean is ca. 13 oC • Red Sea can be 21.5 oC @ 2000 m depth • Weddell Sea can be -1.9 oC • Hydrothermal vent effluent can approach 400 oC Pressure • • • 3. Increases predictably by 1 atmosphere (14.7 psi) every 10 m Mean depth of oceans – 3800 m = 5600 psi Affects biological molecules – Membranes, enzymes Light • • • Decreases with depth Sunlight present in mesopelagic zone; absent below 1000 m Affects development of eyes I. Deep Sea A. Conditions 4. Dissolved Oxygen • • 5. Near saturation and not limiting in most of the deep sea • Exceptions: OMZ and certain enclosed basins (Santa Barbara Basin, Cariaco Basin, Black Sea) OMZ and anoxic basins may act as barriers Substrate • • Exposed hard rock is uncommon • Biogenic hard substrate may be important Sediment is common • Continental margins – coarse terrigenous material • Deep-sea floor – biogenic oozes, terrigenous clays • Deep-sea sediments typically very low in organic carbon – 0.5% beneath productive areas and <0.1% beneath oligotrophic waters Oxygen Minimum Zone (OMZ) Oxygen Minimum Zone (OMZ) How do OMZ species adapt to low levels of oxygen? •Metabolic rate (O2 consumption) declines •Gill ventilation rates increase •Hemoglobin binds oxygen at lower saturation •Gene expression: enzyme isoforms for anaerobiosis •Some may be food-deprived Oxygen Minimum Zone (OMZ) I. Deep Sea A. 4. 5. Conditions • • • • • Dissolved Oxygen Near saturation and not limiting in most of the deep sea • Exceptions: OMZ and certain enclosed basins (Santa Barbara Basin, Cariaco Basin, Black Sea) OMZ and anoxic basins may act as barriers Substrate Most of deep sea floor covered by sediments • Margins – Coarse terrigenous sediments • Basins – Biogenic oozes (>30% biogenic skeletal material) and terrigenous clays (depth related) • Siliceous oozes – Diatoms (high latitudes) or radiolarians (tropics) • Calcareous oozes – Foraminiferans (productive areas) Low organic content (typically <1%) Exposed hard substrate uncommon • Rocks, manganese nodules, biogenic I. Deep Sea A. Conditions 6. Currents • • • • 7. Generally slow – Mean speeds typically <5 cm s-1, with peaks less than 20 cm s-1 in most areas Periodically, certain areas experience benthic storms • Typically last days to weeks Tidal currents Source of temporal and spatial variability Food Supply • • • 8. Variable in time and space Seasonal variation • Seasonality in productivity, migration patterns, storms, etc. • May produce seasonal patterns in biological processes (Ex: behavior, feeding, metabolism, reproduction, recruitment) Episodic large inputs may introduce variability on other time and space scales Trends • • Gigantism – Ex: Xenophyophores, Amphipods, Isopods Miniaturization – Ex: Ostracods, Tanaids, Harpacticoid Copepods Philippine Trench Hirondellea gigas – Scavenging Amphipods I. Deep Sea A. Conditions 6. Currents • • • • 7. Generally slow – Mean speeds typically <5 cm s-1, with peaks less than 20 cm s-1 in most areas Periodically, certain areas experience benthic storms • Typically last days to weeks Tidal currents Source of temporal and spatial variability Food Supply • • • 8. Variable in time and space Seasonal variation • Seasonality in productivity, migration patterns, storms, etc. • May produce seasonal patterns in biological processes (Ex: behavior, feeding, metabolism, reproduction, recruitment) Episodic large inputs may introduce variability on other time and space scales Trends • • Gigantism – Ex: Xenophyophores, Amphipods, Isopods Miniaturization – Ex: Ostracods, Tanaids, Harpacticoid Copepods I. Deep Sea B. Fauna • • Most animal phyla present Total faunal abundance decreases sharply with depth • • • Pelagic community biomass at 4000 m ca. 1% of surface values Sinking food accumulates at interfaces (e.g. sediment surface) • Pelagic biomass 10 mab double that at 200 mab (Wishner) Changes in relative abundance of faunal taxa with depth • Kurile-Kamchatka Trench - Sponges dominant component of benthic macro-/megafauna to 2000 m • Holothuroids important below 2000 m and dominant below 8000 m • Asteroids important to 7000 m and absent below that I. Deep Sea B. Fauna • Trophic modes • • • Detritivores and scavengers dominant • Good chemosensory capabilities • Distensible guts Predators relatively uncommon Opportunistic feeding strategies especially useful • Why? Scavengers converge on a food fall 2000m deep off coast of Mexico http://news.bbc.co.uk/1/hi/sci/tech Dec 11 2006 I. Deep Sea B. Fauna • • • • • • Fishes relatively scarce and modified to various degrees, compared to shallow living relatives Typically have reduced or large eyes, watery tissues, low muscle protein content, reduced skeletons, oil-filled swim bladders, body forms not designed for rapid swimming Most important mobile scavengers in deep sea, along with amphipods & isopods Many apparently find food using olfaction Some sit-and-wait predators (e.g. Bathypterois) Some nomadic foragers (e.g. Coryphaenoides) Coryphaenoides Bathypterois Lycodes I. Deep Sea B. Fauna • Whale skull Sessile organisms may be attached to hard substrate of many types – – – • Exposed rock Manganese nodules or bits of geological material Biogenic hard substrate (sponges, shells, wood, bone) Occurrence limited by – – Available substrate Flux of POM (food) Crinoids Antipatharians Gorgonians Barnacle Brachiopods Bryozoan Stalked tunicate I. Deep Sea C. Diversity • Through 1960s, deep sea perceived as highly uniform and consistent over time/space • • Prevailing ecological theory predicted that spatial and temporal uniformity plus sparse, low-grade food resources should lead to an equilibrium condition with a few competitively dominant species Mid-1960s: epibenthic sled developed and deployed by Howard Sanders and Bob Hessler (WHOI) • • Covered much smaller area than conventional deepsea bottom trawl but sampled upper few cm of sediments and retained organisms in a fine-meshed sampling bag Samples effectively ended notion of low diversity in deep sea I. Deep Sea C. Diversity • Number of spp. within many taxa (e.g. bivalves, gastropods, polychaetes) tends to increase from surface to mid-slope depths (ca. 2000 m) then decrease with increasing depth I. Deep Sea C. Diversity • • Trend suggests low species diversity in deep sea Pattern could be artifact of reduced sampling effort with increasing depth • How do we know if we’ve sampled enough area and organisms to generate a meaningful picture of the actual diversity of the deep-sea benthic community? I. Deep Sea C. Diversity • Rarefaction curves for most deep-sea habitats never approach an asymptote Largest quantitative data set to date for deepsea macro- and meiofauna was obtained during early 1980s from Atlantic slope off US • • • • 554 box cores (30 x 30 cm) from depths to 3000 m Over 1600 species identified Factoring out depth, 233 cores taken at 2100 m depth along 176-km long transect • Samples: 798 species from 14 invertebrate phyla I. Deep Sea C. Diversity • Rarefaction curves for most deep-sea habitats never approach an asymptote • Expected number of species increasing at about 25 m-2 • • Prediction: 5-10 million species in deep sea!! No single species >8% of community • Similar to other deep-sea sites (except HEBBLE, where single species may be 50-64% of community) I. Deep Sea C. Diversity 1. Patterns • • Deep-sea species diversity differs among ocean basins • Differences may be related to oxygen content, nutritional input, geological history, etc. High species diversity may be due to 1) Processes that establish diversity (speciation) 2) Process that maintain diversity (extinction) I. Deep Sea C. Diversity 2. Maintenance a) Equilibrium processes • Ex: Resource partitioning, habitat partitioning • Species that are well-adapted to a particular set of conditions co-exist at densities near carrying capacity of environment b) Disequilibrium processes • Ex: Local disturbance • Patchy habitat supports many populations at early growth stages, hence at relatively low densities (not near carrying capacity), reducing competitive exclusion as an important structuring mechanism • Connell (1978) suggested that highest diversity maintained at intermediate levels of disturbance Hydrothermal Vents Hydrothermal Vent fluids: Acidic (pH 2.8), Hydrogen Sulfide >1mM Temperature up to 400°C Chemosynthetic Food Web: Sulfide Oxidizing Bacteria Riftia pachyptila (2 m tall) Fine-scale adaptation to thermal niches Distribution patterns at the vents. Black Smoker Warm vent Alvinella pompejana & A. caudata Bythograea thermydron Cool vent Deep Sea- Vent H2S 0->1mM Temp 2-400°C pH 8 -2.8 Calyptogena magnifica Cool vent Riftia pachyptila Bathymodiolus thermophilus Vents are short-lived. Seamounts have higher biomass and different communities Seamount Food Webs Vertical migrators move to regions with more food Swept over seamounts by currents Trapped on top at dawn Abundance of predators high, musculature robust, but SLOW growth What types of adaptations are needed to support life at depth? • Tolerance Adaptations: adapt to perturbation • from abiotic conditions, e.g., hydrostatic pressure and temperature Capacity Adaptations: adjust rates of life in accord with the abiotic and biotic conditions ‘Rate of living’ falls for visual predators, but not for gelatinous ‘float and wait’ predators. For review, see: Childress, J.J. (1995). Trends Ecol. Evol. 10: 30-36 “Float-and-wait” feeding may become more important than intense predation with reduced visual predation Capacity Adaptations: conclusions •Reduced intensity of locomotory activity less reliance on visual predation = lower metabolic capacity. •Reduced muscle protein levels = lower costs of maintenance metabolism & growth •Lower O2 consumption •Reduced/Absent swim bladders; reduced calcification •Migrators and Non-Migrators differ Tolerance Adaptations: Pressure & Temperature • Adaptive Solutions: a cooperative venture between macroand ‘micro’molecules. Proteins: amino acid substitutions – Enhance flexibility – Conserve Km (substrate binding) at habitat pressure Osmolytes: protein-stabilizing solutes Lipids & membranes: fluidity-effects – Homeoviscous adaptation More unsaturated acyl phospholipid chains Gas-filled spaces—obvious problems V = nRT/P PRESSURE EFFECTS IN THE LIQUID PHASE— PROTEIN conformational changes a problem! •Movement during substrate binding/release •Subunit polymerization Lactate Dehydrogenase (LDH) Pyruvate + NADH + H+ lactate + NAD+ Pressure inhibits membrane-spanning proteins: resistance to conformational change. Membrane-spanning protein Conformational change Low resistance—high activity High resistance--inhibition Homeoviscous Adaptation Shifts in acyl chain ‘saturation’ (double-bond content: =) saturated mono-unsaturated Viscous poly-unsaturated Fluid Homeoviscous adaptation Viscosity Change lipid composition (saturation of fatty acid side chains, cholesterol) Maintain stable fluidity at habitat conditions Preserve membrane permeability and membrane enzyme function A B A C B C Temperature (°C) Pressure (atms)