* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 11 - Stars and Atomic Spectra
Dyson sphere wikipedia , lookup
International Ultraviolet Explorer wikipedia , lookup
Aries (constellation) wikipedia , lookup
Cassiopeia (constellation) wikipedia , lookup
Auriga (constellation) wikipedia , lookup
Cygnus (constellation) wikipedia , lookup
Canis Minor wikipedia , lookup
Corona Borealis wikipedia , lookup
Cosmic distance ladder wikipedia , lookup
Corona Australis wikipedia , lookup
Observational astronomy wikipedia , lookup
Perseus (constellation) wikipedia , lookup
Star catalogue wikipedia , lookup
Canis Major wikipedia , lookup
Type II supernova wikipedia , lookup
Future of an expanding universe wikipedia , lookup
Timeline of astronomy wikipedia , lookup
Aquarius (constellation) wikipedia , lookup
H II region wikipedia , lookup
Stellar kinematics wikipedia , lookup
Stellar evolution wikipedia , lookup
Star formation wikipedia , lookup
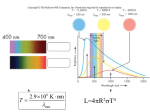
Stellar Spectra • • • • • • • Colors/spectra of stars Classifying stars Photons Atomic structure Elements in stars Masses of stars Mass-luminosity relation Reading: sections: 19.5, 19.7-19.9, 5.4-5.8 A star’s color depends on its surface temperature Stars are assigned a `spectral type’ based on their spectra • The spectral classification essentially sorts stars according to their surface temperature. • The spectral classification also uses spectral lines. Spectral type • Sequence is: O B A F G K M • O type is hottest (~25,000K), M type is coolest (~2500K) • Star Colors: O blue to M red • Sequence subdivided by attaching one numerical digit, for example: F0, F1, F2, F3 … F9 where F1 is hotter than F3 . Sequence is O … O9, B0, B1, …, B9, A0, A1, … A9, F0, … • Useful mnemonics to remember OBAFGKM: – Our Best Astronomers Feel Good Knowing More – Oh Boy, An F Grade Kills Me – (Traditional) Oh, Be a Fine Girl (or Guy), Kiss Me Classifying stars • We now have two properties of stars that we can measure: – Luminosity – Color/surface temperature • Using these two characteristics has proved extraordinarily effective in understanding the properties of stars – the Hertzsprung-Russell (HR) diagram HR diagram HR diagram • Originally, the HR diagram was made by plotting absolute magnitude versus spectral type • Now, it’s better to think of the HR diagram in terms of physical quantities: luminosity and surface temperature If we plot lots of stars on the HR diagram, they fall into groups These groups indicate types of stars, or stages in the evolution of stars Luminosity classes • • • • • Class Ia,b : Supergiant Class II: Bright giant Class III: Giant Class IV: Sub-giant Class V: Dwarf The Sun is a G2 V star The spectrum of a star is primarily determined by 1. 2. 3. 4. The temperature of the star’s surface The star’s distance from Earth The density of the star’s core The luminosity of the star Photon energy • Up to now, we have been discussing the wavelength of light as determining it color • However, light comes in discrete packets called photons and the energy of each photon is set by its color or wavelength • From Einstein, we known that the photon energy is inversely proportional to its wavelength Photon energy Hydrogen atom Electron orbits around nucleus Electron orbits From quantum mechanics, only certain orbits are allowed. Each orbit has a specific energy. How atoms emit light How atoms emit light • The emitted photon has an energy which is exactly the energy difference between the orbits that the electron had before and after. • Because only certain energies are allowed for the electron orbits, only certain energies of photons can be produced. We call these the spectral lines of hydrogen. Spectral lines of hydrogen The length of each arrow determines the energy and therefore the wavelength of the photon emitted. Spectral lines • Each element (hydrogen, helium, neon, mercury, iron, …) has its own particular set of energy levels and its own set of spectral lines. • Do demonstration (7B10.10) Uses of spectral lines • Because each element has it own unique pattern of spectral lines, the spectral lines from stars can be used to determine the composition, or the relative number of atoms of each elements, of the stars Kirchhoff’s Laws Absorption spectrum of a star Composition of a typical star Masses of stars • Spectral lines also allow us to measure the velocities of stars via the Doppler shift that we discussed in searching for extra-solar planets. Doppler shift measurements are usually done on spectral lines. • Essentially all of the mass measurements that we have for stars are for stars in binary systems – two stars orbiting each other. • The mass of the stars can be measured from their velocities and the distance between the stars. Mass-Luminosity relation