* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download XIENCE Xpedition WICPA
Remote ischemic conditioning wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Cardiac surgery wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
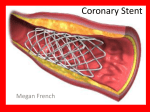
The XIENCE V®, XIENCE nano®, XIENCE PRIME®, and XIENCE PRIME® LL, XIENCE Xpedition™, XIENCE Xpedition™ SV and XIENCE Xpedition™ LL (XIENCE Family) of Everolimus Eluting Coronary Stents on the MULTI-LINK VISION® or MULTI-LINK MINI VISION® Delivery Systems INDICATIONS The XIENCE Family of Everolimus Eluting Coronary Stent Systems are indicated for improving coronary luminal diameter in patients with symptomatic heart disease due to de novo native coronary artery lesions (XIENCE V, XIENCE nano and XIENCE Xpedition SV length ≤ 28 mm and XIENCE PRIME, XIENCE PRIME LL, XIENCE Xpedition and XIENCE Xpedition LL length ≤ 32 mm) with reference vessel diameters of 2.25 mm to 4.25 mm. CONTRAINDICATIONS The XIENCE Family of stents is contraindicated for use in patients: •Whocannotreceiveantiplateletand/oranti-coagulanttherapy •Withlesionsthatpreventcompleteangioplastyballooninflationorproperplacement of the stent or stent delivery system •Withhypersensitivityorcontraindicationtoeverolimusorstructurally-relatedcompounds, cobalt,chromium,nickel,tungsten,acrylic,and/orfluoropolymers. WARNINGS •Ensurethattheinnerpackagesterilebarrierhasnotbeenopenedordamagedpriortouse. •Judiciouspatientselectionisnecessarybecausedeviceusehasbeenassociated with stent thrombosis,vascularcomplications,and/orbleedingevents. •Thisproductshouldnotbeusedinpatientswhoarenotlikelytocomplywiththe recommended antiplatelet therapy. PRECAUTIONS •Stentimplantationshouldonlybeperformedbyphysicianswhohavereceivedappropriate training. •Stentplacementshouldbeperformedathospitalswhereemergencycoronaryartery bypassgraftsurgeryisaccessible. •Subsequentrestenosismayrequirerepeatdilatationofthearterialsegmentcontaining thestent.Long-termoutcomesfollowingrepeatdilatationofthestentarepresently unknown. •Risksandbenefitsshouldbeconsideredinpatientswithseverecontrastagent allergies. •Careshouldbetakentocontroltheguidingcathetertipduringstentdelivery,deployment andballoonwithdrawal.Beforewithdrawingthestentdeliverysystem,visuallyconfirm completeballoondeflationbyfluoroscopytoavoidguidingcathetermovementintothe vesselandsubsequentarterialdamage. •Stentthrombosisisalow-frequencyeventthatisfrequentlyassociatedwith myocardial infarction (MI) or death. •WhenDESareusedoutsidethespecifiedIndicationsforUse,patientoutcomesmay differfromtheresultsobservedintheSPIRITfamilyoftrials. •ComparedtousewithinthespecifiedIndicationsforUse,theuseofDESinpatients andlesionsoutsideofthelabeledindicationsmayhaveanincreasedriskofadverse events,includingstentthrombosis,stentembolization,MI,ordeath. •Orallyadministeredeverolimuscombinedwithcyclosporineisassociatedwithincreased serum cholesterol and triglycerides levels. •Apatient’sexposuretodrugandpolymerisproportionaltothenumberandtotallengthof implanted stents. See Instructions for Use for current data on multiple stent implantation. •SafetyandeffectivenessoftheXIENCEFamilyofstentshavenotbeenestablishedfor subjectpopulationswiththefollowingclinicalsettings: •Patientswithpriortargetlesionorin-stentrestenosisrelatedbrachytherapy,patients in whom mechanical atherectomy devices or laser angioplasty devices are used simultaneously, women who are pregnant or lactating, men intending to father children, pediatricpatients,unresolvedvesselthrombusatthelesionsite,coronaryartery reference vessel diameters < 2.25 mm or > 4.25 mm or lesion length > 32 mm, lesions located in saphenous vein grafts, unprotected left main coronary artery, ostial lesions,chronictotalocclusions,lesionslocatedatabifurcationorpreviouslystented lesions,diffusediseaseorpoorflow(TIMI<1)distaltotheidentifiedlesions,excessive tortuosityproximaltoorwithinthelesion,recentacutemyocardialinfarction(AMI) orevidenceofthrombusintargetvessel,moderateorseverelesioncalcification, multivesseldisease,andin-stentrestenosis •Everolimushasbeenshowntoreducetheclearanceofsomeprescriptionmedications when itwasadministeredorallyalongwithcyclosporine(CsA).Formaldruginteractionstudies havenotbeenperformedwiththeXIENCEFamilyofstentsbecauseoflimitedsystemic exposuretoeverolimuselutedfromthestent. •Everolimusisanimmunosuppressiveagent.Considerationshouldbegiventopatients takingotherimmunosuppressiveagentsorwhoareatriskforimmunesuppression. •Oraleverolimususeinrenaltransplantpatientsandadvancedrenalcellcarcinoma patients was associated with increased serum cholesterol and triglycerides, which in some cases requiredtreatment. •Non-clinicaltestinghasdemonstratedthattheXIENCEFamilyofstents,insingleandin overlappedconfigurationsupto68mminlengthforXIENCEVandXIENCEnanoandup to71mminlengthforXIENCEPRIME,XIENCEPRIMELL,XIENCEXpedition,XIENCE XpeditionSVandXIENCEXpeditionLLareMRConditional.Itcanbescannedsafelyunder the conditions in the Instructions for Use. •TheXIENCEFamilyofstentsshouldbehandled,placed,implanted,andremovedaccording to the Instructions for Use. POTENTIAL ADVERSE EVENTS Adverseevents(inalphabeticalorder)whichmaybeassociatedwithcoronarystentusein nativecoronaryarteriesincludebutarenotlimitedto: •Abruptclosure,Accesssitepain,hematoma,orhemorrhage,Acutemyocardialinfarction, Allergicreactionorhypersensitivitytocontrastagentorcobalt,chromium,nickel, tungsten,acrylicandfluoropolymers;anddrugreactionstoantiplateletdrugsorcontrast agent,Aneurysm,Arterialperforationandinjurytothecoronaryartery,Arterialrupture, Arteriovenousfistula,Arrhythmias,atrialandventricular,Bleedingcomplications,which mayrequiretransfusion,Cardiactamponade,Coronaryarteryspasm,Coronaryorstent embolism,Coronaryorstentthrombosis,Death,Dissectionofthecoronaryartery,Distal emboli(air,tissueorthrombotic),Emergentornon-emergentcoronaryarterybypassgraft surgery,Fever,Hypotensionand/orhypertension,Infectionandpainatinsertionsite, Injurytothecoronaryartery,Ischemia(myocardial),Myocardialinfarction(MI),Nausea andvomiting,Palpitations,Peripheralischemia(duetovascularinjury),Pseudoaneurysm, RenalFailure,Restenosisofthestentedsegmentoftheartery,Shock/pulmonaryedema, Stroke/cerebrovascularaccident(CVA),Totalocclusionofcoronaryartery,Unstable orstableanginapectoris,Vascularcomplicationsincludingattheentrysitewhichmay requirevesselrepair,Vesseldissection Adverseeventsassociatedwithdailyoraladministrationofeverolimustoorgantransplant patientsincludebutarenotlimitedto: •Abdominalpain(includingupperabdominalpain);Anemia;Angioedema;Anorexia; Asthenia;Constipation;Cough;Delayedwoundhealing/fluidaccumulation;Diarrhea; Dyslipidemia(includinghyperlipidemiaandhypercholesterolemia);Dyspnea;Dysgeusia; Dyspepsia;Dysuria;Dryskin;Edema(peripheral);Epistaxis;Fatigue;Headache;Hematuria; Hyperglycemia(mayincludenewonsetofdiabetes);Hyperlipidemia;Hyperkalemia; Hypertension;Hypokalemia;Hypomagnesemia;Hypophosphatemia;Increasedserum creatinine;Infectionsandseriousinfections:bacterial,viral,fungal,andprotozoal infections(mayincludeherpesvirusinfection,polyomavirusinfectionwhichmaybe associatedwithBKvirusassociatednephropathy,and/orotheropportunisticinfections); Insomnia;InteractionwithstronginhibitorsandinducersofCY3PA4;Leukopenia; Lymphomaandothermalignancies(includingskincancer);Maleinfertility(azospermia and/oroligospermia);Mucosalinflammation(includingoralulcerationandoralmucositis); Nausea;Neutropenia;Non-infectiouspneumonitis;Pain:extremity,incisionsiteand procedural,andback;Proteinuria;Pruritus;Pyrexia;Rash;Stomatitis;Thrombocytopenia; Thromboticmicroangiopathy(TMA)/Thromboticthrombocytopenicpurpura(TTP)/Hemolytic uremicsyndrome(HUS);Tremor;Urinarytractinfection;Upperrespiratorytractinfection; Vomiting •Livevaccinesshouldbeavoidedandclosecontactwiththosethathavehadlivevaccines shouldbeavoided.Fetalharmcanoccurwhenadministeredtoapregnantwoman.There maybeotherpotentialadverseeventsthatareunforeseenatthistime. Prior to use, please reference the Instructions for Use at www.abbottvascular.com/ifu for more information on indications, contraindications, warnings, precautions, and adverse events. MULTI-LINK VISION, MULTI-LINK MINI VISION, XIENCE V, XIENCE nano, XIENCE PRIME and XIENCE Xpedition are trademarks of the Abbott Group of Companies. ©2012 Abbott. All rights reserved. AP2937829-US Rev. A