* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PDF - The Dermatologist
Survey
Document related concepts
Transcript
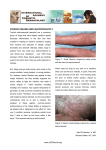
LETTER DEAR DERMATOLOGIST: Welcome to the first issue of the re-designed Derm Practice, a quarterly newsletter for practicing dermatologists. As a fellow dermatologist, I know how busy you are seeing patients while managing and marketing the business end of your practice. This newsletter is designed to include several articles on topics that are timely and relevant to the clinical and practice management sides of your practice. As the Medical Editor of this publication, I will do my best to ensure that the newsletter provides you with what you need to better your practice. I will also author the Expert Advice column in each issue. E-mail me your questions through [email protected]. Michael H. Gold, M.D. A LOOK AT THIS ISSUE In this issue, I answer common questions about buying a laser and selling skin products in the office. I then describe how the use of Levulan® Kerastic® (aminolevulinic acid HCI) and photodynamic therapy (LevulanPDT) has steadily risen over the past several years as more dermatologists realize the benefit of this therapy. Contributing Editor Mike Krivda then explores the problems with compounding aminolevulinic acid (ALA). DUSA has had to bring lawsuits against pharmacists and physicians illegally using compounded ALA in order to protect its patents for Levulan-PDT. This was done to protect the reputation of DUSA’s products from damage that could result from the use of an unsafe copy of its product. This article details the current lawsuits as well as how some leading dermatologists view the issue. I hope you find this issue of Derm Practice valuable. Sincerely, Michael H. Gold, M.D. WELCOME FROM DUSA PHARMACEUTICALS As part of our commitment to the dermatology community, we here at DUSA are excited to sponsor this newsletter so that it can provide you with timely, practical advice in order to help you run the best possible practice. We hope that you find the articles in this issue and future issues valuable and informative. Please e-mail [email protected] with any comments or suggestions for the newsletter. We look forward to hearing from you. Sincerely, Robert F. Doman President and Chief Operating Officer, DUSA Pharmaceuticals, Inc. EXPERT ADVICE THIS ESTABLISHED PRACTITIONER AND CLINICAL AND PRACTICE MANAGEMENT EXPERT ANSWERS YOUR MOST PRESSING QUESTIONS. BY MICHAEL H. GOLD, M.D If I am in the market for a laser or light source, which one should I buy? Q: We are very fortunate to have a great deal of lasers and light sources at our disposal and lots of companies manufacturing these devices that want our business. First, find a company and a sales representative you are comfortable with, and a representative who truly knows his or her product and who can promote it without putting down every other device in the same product range. These devices all work similarly, with subtle differences that the representative should be able to share with you without putting down the competition. Sales representatives who like to put down their competition are not welcome in my clinic. Also, speak with other physicians who utilize these devices; but keep in mind that most of us are consultants to a variety of medical device companies and will tell you the advantages of a specific device. Most importantly, find out the repair/warranty information from the company as you cannot afford to have these devices be “down” for a long period. If the representative does not take an active role in your practice and help ensure your success, look elsewhere. A: VERSATILE SYSTEMS I think every practice should own an intense pulsed light (IPL) system, which is one of the most versatile devices on the market. Personally, I like the Lumenis One, the Sciton BBL, the Cynosure PhotoSilk and the Alma Harmony, but there are many IPL systems available on the market. Review them all before making a decision. New devices are also available for the treatment of vascular lesions, with new combination devices becoming available. Furthermore, newer near-infrared collagen remodeling devices and radio frequency devices are adding to the armamentarium we now have to treat the skin non-ablatively with minimal to no downtime. We’ll cover these devices in a future issue. If you advertise in a newspaper to showcase a certain skincare product, that company can help you with the cost. How do I get skincare product companies to help me sell their products? Q: Dispensing skincare products from our offices has become the norm amongst many of our colleagues in dermatology. When you’re selling products in your office, the companies that own these products should work with you since you’re paying them to promote their products. They should not get your business for nothing. A: CO-OP DOLLARS There are several simple initiatives that you can use to get help from 3 these companies in promoting their products. Most companies have coop dollars available. That is, for every product you purchase, a certain percentage of the purchase is put into a “pot” for you to use for advertising or for discounts on future purchases. Sometimes, companies will help you with co-op advertising. If you advertise in a newspaper to showcase a certain product, that company may have funds available to help you with the cost of the advertising. We also ask the companies to provide “gift baskets”, which we use for in-house promotions and for charitable donations. This costs you nothing, yet gives you and the skincare company promotional opportunities. PROMOTIONAL OPPORTUNITIES Skincare companies also can do other events for your office. For instance, one company provided the use of an UV camera system in our medispa so that everyone who came to my office for a 3-day period of time had UV pictures made of their skin. Then, we, along with the company representative who remained with us during the promotion, were able to show the patients their UV damage and make “on-the-spot” recommendations for their skin care. Companies will work with us. It’s in their best interests to be advocates for our practices. But remember, if you do not ask, you will not receive. We will cover office dispensing in a future issue of Derm Practice. ■ Dr. Gold is Director of the Gold Skin Care Center and the Tennessee Clinical Research Center in Nashville, TN. He is also Clinical Assistant Professor in the Department of Medicine, Division of Dermatology at Vanderbilt University School of Medicine and School of Nursing in Nashville, TN. THE GROWING ROLE OF LEVULAN-PDT H O W T H I S C O M B I N AT I O N I S T R E AT I N G A K S A N D M O R E . BY MICHAEL H. GOLD, M.D. he use of aminolevulinic acid and photodynamic therapy (ALA-PDT) by dermatologists has steadily risen over the past several years as more of us realize the benefit that this therapy brings to our patients is outstanding. In the United States, the first indication for ALA was for the treatment of non-hyperkeratotic AKs of the face and scalp with a blue light source after a 14- to 18-hour drug incubation for 16 minutes and 40 seconds in the blue light. T PROVEN RESULTS The ALA utilized most frequently in the United States is a 20% 5-ALA (Levulan® Kerastick®) and the blue light studied is the Blu-U Illuminator, both of which are manufactured by DUSA Pharmaceuticals. The pivotal Phase II clinical trial was reported in 2001.1 In this trial, 36 individuals were evaluated after treatment of their AKs with Levulan and a Blu-U light source with drug incuba- tion time of 14 to 18 hours. In this study, 66% of individual AKs responded with one treatment; 85% of the AKs cleared with two treatments 8 weeks apart. The Phase III clinical trial looked at 243 individuals in a multi-center setting.2 It also utilized individualized AK treatment and drug incubation of 14 to 18 hours and a blue light source. The study showed >70% of the AKs were cleared with one treatment at 12 weeks and 88% after two treatments. This was statistically superior to a placebo arm. As a secondary endpoint of the clinical trial, 94% of patients noted a cosmetic effect after the therapy, a rejuvenation-type of effect with improved skin texture. Even with these results, physicians did not “buy” into this therapy for a variety of reasons. This included predominantly the long drug incubation time, which required patients to have two consecutive day visits to our clinics, something with which our patients and the medical community weren’t comfortable. Also, patients complained of a great deal of pain with the associated light therapy. The treatments caused patients to have “downtime” — up to a week of healing, another factor patients did not want and that cosmetic dermatologists weren’t comfortable with either. BETTER TREATMENT GUIDELINES From these initial clinical trials and in an attempt to determine an easier and more acceptable method for all dermatologists to adopt this therapy into their own practices, a group of individuals began research initiatives exploring the idea of utilizing “shortcontact, full-face” ALA therapy with blue light and other light sources. These lasers and light sources were already known to work within the spectrum of light for protoporphyrin IX (See Figure 1), which is the active form of Levulan once it’s absorbed into the skin. These light sources predominantly included the long-pulsed pulsed dye lasers (PDLs), KTP vascular lasers and the intense pulsed light sources (IPLs). ALA AND IPL The early studies looked at the blue light and the IPL in the treatment of AKs and photorejuvenation. Touma and Gilchrest3 showed blue light improved the skin’s sallowness, fine wrinkling and mottled hyperpigmentation with similar efficacy using 1-hour drug incubation as compared to the 14- to 18-hour drug incubation. Their study, pivotal for the “new” ALA-PDT treatment paradigm, showed statistically no difference between 1-, 2- and 3-hour drug incubation when compared to the 14- to 18-hour drug incubation time. The first use of an IPL with ALA, as Figure 1: A variety of other light sources have also been used to activate ALA-induced PpIX 4 reported by Ruiz-Rodriquez, et al.4 was also shown to be useful in reducing signs of photodamage as well as clearing 87% of the associated AKs in one of the early ALA/IPL clinical trials. Subsequent researchers were able to verify the blue light and IPL results with ALA. This author5 used three monthly ALA-PDT treatments to evaluate photoaging and associated AKs in 10 individuals. Drug incubation time was from 30 to 60 minutes. At the conclusion of the trial (3-month follow-up from the last ALA-IPL treatment), 83% of the AKs were found to have cleared and all the parameters of photorejuvenation improved: crow’s feet 90%, tactile skin roughness 100%, mottled hyperpigmentation 90% and facial erythema 70%. Others looked at similar types of clinical research. Goldman, et al.6 studied 32 patients with moderate photodamage and multiple AKs. Using a 1-hour drug incubation and full-face therapy, they were able to demonstrate a 90% response rate in AK clearance and improvement in photorejuvenation (skin texture 72%, pigment change 59%). More than two-thirds of patients preferred ALAPDT over cryotherapy. Avram et al.7 used an IPL and short-contact drug therapy in a group of individuals with photodamage and AKs. Following a 1-hour drug incubation, 69% of AKs cleared with one treatment. Also, they found photorejuvenation changes including improvement in telangectasia (55%), pigment changes (48%), and positive changes in skin texture (25%). ALA AND OTHER LASERS Alexiades-Armenakas et al.8 evaluated 3- and 18-hour drug incubation using PDL in treating more than 2,500 AKs on and off the face. They found the shorter drug incubation was as effective in AK clearance as the long incubation; clearance rates of 90% on the face and 74% on the torso were reported with the shortcontact ALA. A more recent report by the same group9 evaluated the PDL in the treatment of actinic chelitis with short-contact ALA. Nineteen individuals were treated with ALA and PDL; a 68% clearance was noted at the 12-month follow-up. The KTP laser has also been reported to be useful in photorejuvenation with ALA.10 SPLIT-FACE ANALYSIS To further evaluate the treatment of AKs/photorejuvenation with light sources, several investigations have been performed evaluating split-face analyses comparing IPL or PDL on Patient was treated with ALA-PDT for 15 to 30 minutes before IPL therapy at 550 nm to 570 nm, double pulsing, 3.5 ms pulse durations and fluences of 34 J/cm2 once a month for 3 months. Actinic keratosis improved, as well as skin texture and sallowness. one side of the face to the other side treated with both ALA and an IPL or PDL device. Alster, et al.11 reported the first split-face trial in 2005. Ten patients, with mild-to-moderate photodamage received two split-face ALA-IPL treatments on one side of the face and IPL only on the other side. Clinical improvement scores were consistently higher on the ALA-IPL treated side as compared to the IPL alone side. Key12 reported the use of the PDL in a split-face clinical trial. The ALAPDL treated side showed more improvement than PDL alone. Marmur, et al.13 looked at ultrastructural changes in a group of seven patients who received ALA-IPL on one side of the face and IPL alone on the other side. An increase in Type I collagen was noted in all individuals, but was more so on the ALAIPL treated side. Bhatia, et al.14 reported their spiltface ALA-IPL study recently. They found the ALA-IPL treated areas had greater global scores for photoaging, mottled hyperpigmentation, and in fine lines compared to IPL alone. This group also found split-face ALA-IPL therapy to be superior to IPL alone in treating photoaging and associated AKs.15 There were more improvements noted in crow’s feet, tactile skin roughness, mottled hyperpigmentation, facial erythema and AK clearance in the ALA-IPL sides versus the IPL treated areas. A FIRST-LINE THERAPY ALA-PDT therapy is here to stay. Phase II clinical trials for photorejuvenation are underway, and hopefully we will see an FDA clearance for this indication in the future. In the meantime, the use of ALA-PDT in the treatment of AKs utilizing short-contact, full-face therapy should be considered as first line for all individuals seeking treatment from us for these clinical concerns. ■ 5 Dr. Gold is Director of the Gold Skin Care Center and the Tennessee Clinical Research Center in Nashville, TN. He is also Clinical Assistant Professor in the Department of Medicine, Division of Dermatology at Vanderbilt School of Medicine and School of Nursing in Nashville, TN. References: 1. Jeffes EW, McCullough JL, Weinstein GD, Kaplan R, Glazer SD, Taylor JR. Photodynamic therapy of actinic keratoses with topical aminolevulinic acid hydrochloride and fluorescent blue light. J Am Acad Dermatol 2001;45:96-104. 2. Piacquadio DJ, Chen DM, Farber HF, Fowler JF Jr, Glazer SD, Goodman JJ, Hruza LL, Jeffes EW, Ling MR, Phillips TJ, Rallis TM, Scher RK, Taylor CR, Weinstein GD. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol 2004;140(1):41-6. 3. Touma D, Yaar M, Whitehead S et al. A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch Dermatol 2004;140:33-40. 4. Ruiz-Rodriquez R, Sanz-Sanchez T, Cordobo S. Photodynamic photorejuvenation. Dermatol Surg 2002;28:742-744. 5. Gold MH, Goldman MP. 5-Aminolevulinic Acid Photodynamic Therapy: Where we have been and where we are going. Derm Surg 2004;30:1077-1084. 6. Goldman MP, Atkin D, Kincad S. PDT/ALA in the treatment of actinic damage: real world experience. J Lasers Surg Med 2002;14(S):24. 7. Avram DK, Goldman MP. Effectiveness and safety of ALA-IPL in treating actinic keratoses and photodamage. J Drugs Dermatol 2004;3(1 Suppl):S36-9. 8. Alexiades-Armenakas MR, Geronemus RG. Lasermediated photodynamic therapy of actinic keratoses. Arch Dermatol 2003;139(10):1313-20. 9. Alexiades-Armenakas MR, Geronemus RG. Laser-mediated photodynamic therapy of actinic chelitis. J Drugs Dermatol 2004;3(5):548-551. 10. Personal communication, Kauvar 2006. 11. Alster TS, Tanzi EL, Welsh EC. Photorejuvenation of facial skin with topical 20% 5aminolevulinic acid and intense pulsed light treatment: A split-face comparison study. J Drugs Dermatol 2005;4:35-38. 12. Key DJ. Aminolevulinic acid-pulsed dye laser photodynamic therapy for the treatment of Photoaging. Cosm Derm 2005;18:31-36. 13. Marmur ES, Phelps R, Goldberg DJ. Ultrastructural changes seen after ALA-IPL photorejuvenation: a pilot study. J Cosmet Laser Ther 2005;7(1):21-4. 14. Bhatia AC, Dover JS et al. Adjunctive use of topical aminolevulinic acid with intense pulsed light in the treatment of photoaging. (Presentation Controversies and Conversations in Cutaneous Laser Surgery, Mt. Tremblant, Canada, August 2004). 15. Dover JS, Bhatia AC, Stewart B, Arndt KA. Topical 5-aminolevulinic acid combined with intense pulsed light in the treatment of photoaging. Arch Dermatol 2005;141(10):1247-52. PROTECTING PATIENTS USA Pharmaceuticals, Inc. manufactures Levulan® Kerastic® (aminolevulinic acid HCl), which was FDA-approved for the treatment of actinic keratoses in September 2000. It is the only form of FDA-approved ALA. To protect the substantial investment of time and money DUSA has made to develop and test Levulan, DUSA has obtained patent protection for its therapies. Since its therapies are based on new uses for ALA, an existing molecule, DUSA has obtained what are known as “method of use” patents. This type of patent gives the owner or licensee the exclusive right to perform the “method” described in the patent. In DUSA’s case, this is performing photodynamic therapy (PDT) with ALA for the treatment of actinic keratosis, acne, basal cell carcinoma and various other dermatologic conditions. DUSA has worked diligently to develop and obtain FDA marketing approval for Levulan,” says D. Geoffrey Shulman, M.D., F.R.C.P.C., DUSA’s Chairman and CEO. “To obtain FDA approval, our products have undergone years of extensive and expensive clinical testing and evaluation to reach the dermatology marketplace. Our products and facilities are subject to ongoing review and inspection by FDA to ensure that we continue to meet their high standards for safety, efficacy, potency and purity. Compounded ALA, which has not been approved for marketing by the FDA, does not have to meet these high standards.” D BASIS FOR LAWSUITS Most physicians purchase ALA for PDT from DUSA, but some buy it from compounding pharmacies. Physicians using compounded ALA and compounding pharmacies promoting patented uses of compounded ALA are committing patent infringement. Compounding pharmacies have been selling ALA without informing physicians about the patent infringement liability for using it for purposes covered by DUSA’s patents. In order to protect its patent rights, in 2004 and in early 2005, DUSA sued the Cosmetic Pharmacy of Tucson, AZ, and New England Compounding Center in Framingham, MA, alleging that these compounding pharmacies were promoting their compounded ALA for use in DUSA’s patented therapies and, therefore, inducing physicians to infringe DUSA’s patents. The Cosmetic Pharmacy presented no defense resulting in a DUSA SUES OVER COMPOUNDING OF ALA. BY MICHAEL S. KRIVDA, CONTRIBUTING EDITOR default judgment in DUSA’s favor and attorney fees were also rewarded to DUSA. The second suit against New England Compounding Center was settled. In November 2005, DUSA filed patent infringement suits against physicians in California, Florida and Tennessee. In early 2006, DUSA filed lawsuits against doctors in California, Michigan and Massachusetts. The suits allege that ALA obtained from sources other than DUSA is being used without license from DUSA to perform treatments in direct infringement of DUSA’s patent(s). PROTECTING OUR PROPERTY We have taken these actions to protect our Levulan franchise from illegal exploitation from pharmacies and others who are promoting and selling ALA for our patented uses and to protect our valued customers from unfair competition from those who infringe our patents,” says Dr. Shulman. “While we value the longstanding relationships we have made with the dermatology community, we have unfortunately also had to take action against physicians because, by selling compounded ALA, which can be used for DUSA’s patented therapies, compounding pharmacies are continuing to provide a means by which physicians take on the risk of patent infringement.” DUSA has obtained consent judgments in five suits with the defendants admitting to infringing DUSA’s patents and agreeing to cease illegal conduct. In one case, the defendant also admitted to illegally using Levulan’s trademark (a violation of the Lanham Act) and agreed to cease this conduct. The two remaining cases are still pending. ASSURING QUALITY CARE Compounding pharmacies can provide a valuable service by delivering customized compounded medications not available through any other source. However, there is a distinction between formulating a 6 unique and otherwise unavailable compound and copying an available medication. “As a dermatologist, I know there’s a good use for compounding pharmacies, but when an FDAapproved product is available, the use of a non-FDA approved compounds presents serious potential risks,” says Mitchel Goldman, M.D., Associate Clinical Professor of Dermatology/Medicine at the University of California, San Diego. “If good patient care is your first concern, then you have absolutely no reason to obtain ALA from any other source than the FDA-approved manufacturer.” Noah Scheinfeld, M.D., J.D., Assistant Professor of Dermatology at Columbia University, New York, agrees, chastising physicians who purchase compounded ALA to save money. “It is an unfortunate state of affairs when a licensed physician buys a compounded medication merely to save money,” he says. “It is unethical when that physician isn’t making his patients aware that he or she is charging patients for the name-brand product and pocketing the difference.” TOO MANY RISKS Dr. Goldman notes that many of these purchases are being made by medical spas whose staffs may not understand the risks they’re taking. “Compounding pharmacies are not as experienced as they used to be as the need for compounding is not nearly as great as it was in the past,” he explains. “Should you really be relying on what may be an inexperienced pharmacist to accurately compound a medication that you can simply buy off the shelf? What risks are you exposing your patients to and what risk are you putting yourself in? It just doesn’t seem worth it to me, just to save a few dollars.” “It’s not really a question of legality to me, that’s for the lawyers to settle; it’s about proper care and knowing that you are doing the best thing for your patients,” says Dr. Scheinfeld. ■ Project2 2/1/06 3:00 PM Page 1 Levulan® Kerastick® (aminolevulinic acid HCI) for Topical Solution, 20% Nursing Mothers: The levels of ALA or its metabolites in the milk of subjects treated with LEVULAN KERASTICK Topical Solution have not been measured. Because many drugs are excreted in human milk, caution should be exercised when LEVULAN KERASTICK Topical Solution is administered to a nursing woman. ADVERSE REACTIONS In Phase 3 studies, no non-cutaneous adverse events were found to be consistently associated with LEVULAN KERASTICK Topical Solution application followed by blue light exposure. INDICATIONS AND USAGE The LEVULAN KERASTICK for Topical Solution plus blue light illumination using the BLU-U® Blue Light Photodynamic Therapy Illuminator is indicated for the treatment of minimally to moderately thick actinic keratoses (Grade 1: slightly palpable, better felt than seen or Grade 2: moderately thick, easily seen and felt) of the face or scalp. Photodynamic Therapy Response: The constellation of transient local symptoms of stinging and/or burning, itching, erythema and edema as a result of LEVULAN KERASTICK Topical Solution plus BLU-U treatment was observed in all clinical studies of LEVULAN KERASTICK for Topical Solution Photodynamic Therapy for actinic keratoses treatment. Stinging and/or burning subsided between 1 minute and 24 hours after the BLU-U Blue Light Photodynamic Therapy Illuminator was turned off, and appeared qualitatively similar to that perceived by patients with erythropoietic protoporphyria upon exposure to sunlight. There was no clear drug dose or light dose dependent change in the incidence or severity of stinging and/or burning. CONTRAINDICATIONS The LEVULAN KERASTICK for Topical Solution plus blue light illumination using the BLU-U Blue Light Photodynamic Therapy Illuminator is contraindicated in patients with cutaneous photosensitivity at wavelengths of 400-450 nm, porphyria or known allergies to porphyrins, and in patients with known sensitivity to any of the components of the LEVULAN KERASTICK for Topical Solution. In two Phase 3 trials, the sensation of stinging and/or burning appeared to reach a plateau at 6 minutes into the treatment. Severe stinging and/or burning at one or more lesions being treated was reported by at least 50% of the patients at some time during treatment. The majority of patients reported that all lesions treated exhibited at least slight stinging and/or burning. Less than 3% of patients discontinued light treatment due to stinging and/or burning. WARNINGS The LEVULAN KERASTICK for Topical Solution contains alcohol and is intended for topical use only. Do not apply to the eyes or to mucous membranes. Excessive irritation may be experienced if this product is applied under occlusion. The most common changes in lesion appearance after LEVULAN KERASTICK for Topical Solution Photodynamic Therapy were erythema and edema. In 99% of active treatment patients, some or all lesions were erythematous shortly after treatment, while in 79% of vehicle treatment patients, some or all lesions were erythematous. In 35% of active treatment patients, some or all lesions were edematous, while no vehicle-treated patients had edematous lesions. Both erythema and edema resolved to baseline or improved by 4 weeks after therapy. LEVULAN KERASTICK Topical Solution application to photodamaged perilesional skin resulted in photosensitization of photodamaged skin and in a photodynamic response. (see Precautions). For Topical Use Only • Not for Ophthalmic Use Brief Summary (For full prescribing information, see physician’s insert) PRECAUTIONS General: During the time period between the application of LEVULAN KERASTICK Topical Solution and exposure to activating light from the BLU-U Blue Light Photodynamic Therapy Illuminator, the treatment site will become photosensitive. After LEVULAN KERASTICK Topical Solution application, patients should Other Localized Cutaneous Adverse Experiences: Table 1 depicts the incidence avoid exposure of the photosensitive treatment sites to sunlight or bright indoor light (e.g., examination and severity of cutaneous adverse events, stratified by anatomic site treated. lamps, operating room lamps, tanning beds, or lights at close proximity) during the period prior to blue light treatment. Exposure may result in a stinging and/or burning sensation and may cause erythema and/or edema of the lesions. Before exposure to sunlight, patients should, therefore, protect treated TABLE 1 Post-PDT Cutaneous Adverse Events - ALA-018/ALA-019 lesions from the sun by wearing a widebrimmed hat or similar head covering of light-opaque material. SCALP FACE Sunscreens will not protect against photosensitivity reactions caused by visible light. It has not been LEVULAN (n=42) LEVULAN (n=139) Vehicle (n=41) determined if perspiration can spread the LEVULAN KERASTICK Topical Solution outside the treatment site to eye or surrounding skin. Application of LEVULAN KERASTICK Topical Solution to perilesional areas of photodamaged skin of the face or scalp may result in photosensitization. Upon exposure to activating light from the BLU-U Blue Light Photodynamic Therapy Illuminator, such photosensitized skin may produce a stinging and/or burning sensation and may become erythematous and/or edematous in a manner similar to that of actinic keratoses treated with LEVULAN PDT. Because of the potential for skin to become photosensitized, the LEVULAN KERASTICK for Topical Solution should be used by a qualified health professional to apply drug only to actinic keratoses and not perilesional skin. Degree of Severity Scaling/ Crusting Pain The LEVULAN KERASTICK for Topical Solution has not been tested on patients with inherited or Tenderness acquired coagulation defects. Itching Information for Patients: Edema LEVULAN Photodynamic Therapy for Actinic Keratoses. The first step in LEVULAN KERASTICK photodyUlceration namic therapy (PDT) for actinic keratoses is application of the LEVULAN KERASTICK for Topical Solution Bleeding/ to actinic keratoses located on the patient’s face or scalp. After LEVULAN KERASTICK for Topical Solution is applied to the actinic keratoses in the doctor’s office, the patient will be told to return the Hemorrhage next day. During this time the actinic keratoses will become sensitive to light (photosensitive). Care Hypo/hypershould be taken to keep the treated actinic keratoses dry and out of bright light. After LEVULAN KERApigmentation STICK Topical Solution is applied, it is important for the patient to wear light-protective clothing, such as a wide-brimmed hat, when exposed to sunlight or sources of light. Fourteen to eighteen hours after Vesiculation application of LEVULAN KERASTICK Topical Solution the patient will return to the doctor’s office to Pustules receive blue light treatment, which is the second and final step in the treatment. Prior to blue light Oozing treatment, the actinic keratoses will be rinsed with tap water. The patient will be given goggles to Dysesthesia wear as eye protection during the blue light treatment. The blue light is of low intensity and will not heat the skin. However, during the light treatment, which lasts for approximately 17 minutes, the Scabbing patient will experience sensations of tingling, stinging, prickling or burning of the treated lesions. These feelings of discomfort should improve at the end of the light treatment. Following treatment, the Erosion Excoriation actinic keratoses and, to some degree, the surrounding skin, will redden, and swelling and scaling may also occur. However, these lesion changes are temporary and should completely resolve by 4 Wheal/Flare weeks after treatment. Skin disorder Photosensitivity NOS After LEVULAN KERASTICK Topical Solution is applied to the actinic keratoses in the doctor’s office, the patient should avoid exposure of the photosensitive actinic keratoses to sunlight or bright indoor light (e.g., from examination lamps, operating room lamps, tanning beds, or lights at close proximity) during the period prior to blue light treatment. If the patient feels stinging and/or burning on the actinic keratoses, exposure to light should be reduced. Before going into sunlight, the patient should protect treated lesions from the sun by wearing a wide-brimmed hat or similar head covering of light-opaque material. Sunscreens will not protect the patient against photosensitivity reactions. If for any reason the patient cannot return for blue light treatment during the prescribed period after application of LEVULAN KERASTICK Topical Solution (14 to 18 hours), the patient should call the doctor. The patient should also continue to avoid exposure of the photosensitized lesions to sunlight or prolonged or intense light for at least 40 hours. If stinging and/or burning is noted, exposure to light should be reduced. Drug Interactions: There have been no formal studies of the interaction of LEVULAN KERASTICK for Topical Solution with any other drugs, and no drug-specific interactions were noted during any of the controlled clinical trials. It is, however, possible that concomitant use of other known photosensitizing agents such as griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulfonamides and tetracyclines might increase the photosensitivity reaction of actinic keratoses treated with the LEVULAN KERASTICK for Topical Solution. Carcinogenesis, Mutagenesis, Impairment to Fertility: No carcinogenicity testing has been carried out using ALA. No evidence of mutagenic effects was seen in four studies conducted with ALA to evaluate this potential. In the Salmonella-Escherichia coli/mammalian microsome reverse mutation assay (Ames mutagenicity assay), no increases in the number of revertants were observed with any of the tester strains. In the Salmonella-Escherichia coli/mammalian microsome reverse mutation assay in the presence of solar light radiation (Ames mutagenicity assay with light), ALA did not cause an increase in the number of revertants per plate of any of the tester strains in the presence or absence of simulated solar light. In the L5178Y TK± mouse lymphoma forward mutation assay, ALA was evaluated as negative with and without metabolic activation under the study conditions. PpIX formation was not demonstrated in any of these in vitro studies. In the in vivo mouse micronucleus assay, ALA was considered negative under the study exposure conditions. In contrast, at least one report in the literature has noted genotoxic effects in cultured rat hepatocytes after ALA exposure with PpIX formation. Other studies have documented oxidative DNA damage in vivo and in vitro as a result of ALA exposure. No assessment of effects of ALA HCl on fertility has been performed in laboratory animals. It is unknown what effects systemic exposure to ALA HCl might have on fertility or reproductive function. Pregnancy Category C: Animal reproduction studies have not been conducted with ALA HCl. It is also not known whether LEVULAN KERASTICK Topical Solution can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. LEVULAN KERASTICK Topical Solution should be given to a pregnant woman only if clearly needed. Vehicle (n=21) Mild/ Mild/ Mild/ Mild/ Moderate Severe Moderate Severe Moderate Severe Moderate Severe 71% 1% 12% 0% 64% 2% 19% 0% 1% 1% 25% 1% 4% 4% 0% 0% 1% 0% 0% 0% 0% 0% 7% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 2% 14% 0% 2% 2% 0% 0% 7% 0% 0% 0% 0% 0% 19% 0% 0% 0% 0% 0% 0% 0% 0% 0% 22% 4% 4% 1% 2% 2% 14% 1% 7% 5% 20% 0% 0% 0% 0% 1% 1% 0% 1% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 36% 0% 0% 0% 0% 0% 0% 0% 0% 0% 5% 0% 0% 0% 0% 2% 0% 2% 12% 33% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 0% 5% 0% 0% 0% 0% 0% 0% 0% 0% 0 Adverse Experiences Reported by Body System: In the Phase 3 studies, 7patients experienced a serious adverse event. All were deemed remotely or not related to treatment. No clinically significant patterns of clinical laboratory changes were observed for standard serum chemical or hematologic parameters in any of the controlled clinical trials. OVERDOSAGE LEVULAN KERASTICK Topical Solution Overdose: LEVULAN KERASTICK Topical Solution overdose have not been reported. In the unlikely event that the drug is ingested, monitoring and supportive care are recommended. The patient should be advised to avoid incidental exposure to intense light sources for at least 40 hours. The consequences of exceeding the recommended topical dosage are unknown. BLU-U® Light Overdose: There is no information on overdose of blue light from the BLU-U Blue Light Photodynamic Therapy Illuminator following LEVULAN KERASTICK Topical Solution application. HOW SUPPLIED The LEVULAN KERASTICK for Topical Solution, 20%, is a single-unit dosage form, supplied in packs of 6. Each LEVULAN KERASTICK for Topical Solution applicator consists of a plastic tube containing two sealed glass ampules and an applicator tip. One ampule contains 1.5 mL of solution vehicle. The other ampule contains 354 mg of aminolevulinic acid HCl. The applicator is covered with a protective cardboard sleeve and cap. Product Package Individual LEVULAN KERASTICK for Topical Solution, 20% Carton of 6 LEVULAN KERASTICKS for Topical Solution, 20% NDC number 67308-101-01 67308-101-06 Storage Conditions: Store between 20º– 25ºC (68º– 77ºF); excursions permitted to 15º– 30ºC (59º– 86ºF) [See USP Controlled Room Temperature]. The LEVULAN KERASTICK for Topical Solution should be used immediately following preparation (dissolution). Solution application must be completed within 2 hours of preparation. An applicator that has been prepared must be discarded 2 hours after mixing (dissolving) and a new LEVULAN KERASTICK for Topical Solution used, if needed. LEVULAN®, KERASTICK®, BLU-U®, DUSA Pharmaceuticals, Inc.® and DUSA® are registered trademarks of DUSA Pharmaceuticals, Inc.® US Patents: 5,079,262, 5,211,938, 5,422,093, 5,954,703, 6,710,066 Manufactured for: DUSA Pharmaceuticals, Inc.® 25 Upton Drive, Wilmington, MA 01887 For more information please contact: 1-877-533-3872 or 1-978-657-7500 www.dusapharma.com 7 MKT-1330 Rev B Levulan ® Photodynamic Therapy Treats AKs without weeks of red, raw skin. Instead, skin response usually subsides within a week after treatment, which is why 4 out of 5 patients prefer Levulan to previous 5-FU treatments.3 The BLU-U® Blue Light Photodynamic Therapy Illuminator is also FDA cleared for light alone treatment of moderate inflammatory acne. Levulan® Kerastick® (aminolevulinic acid HCI) for Topical Solution, 20% Levulan® is the only FDA approved ALA Call DUSA at 877-533-3872 or www.dusapharma.com Reference: 1. Data from Phase III Clinical Trials. Data on file, DUSA Pharmaceuticals Inc®. 2.Data based on 173 patients treated and assessed for cosmetic results and physician evaluation of 1340 lesions. 3.Data based on 27 patients who had previous 5-fluorouracil treatment 83 General Warren Blvd., Suite 100 Malvern, PA 19355 94% of patients rated cosmetic response as good to excellent1, 2