* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Frontotemporal dementia – Differentiation from Alzheimer`s disease
Dissociative identity disorder wikipedia , lookup
History of psychiatric institutions wikipedia , lookup
History of psychiatry wikipedia , lookup
Mental status examination wikipedia , lookup
Classification of mental disorders wikipedia , lookup
Creutzfeldt–Jakob disease wikipedia , lookup
Emil Kraepelin wikipedia , lookup
Parkinson's disease wikipedia , lookup
Controversy surrounding psychiatry wikipedia , lookup
Dementia with Lewy bodies wikipedia , lookup
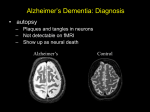
PSYCHOGERIATRIA POLSKA 2004;1(4),279-292 artykuł przeglądowy review article Frontotemporal dementia – Differentiation from Alzheimer’s disease Diagnostyka różnicowa otępienia czołowo-skroniowego i choroby Alzheimera Lars Gustafson, Christina Elfgren, Ulla Passant Department of Psychogeriatrics, University Hospital, Lund, Sweden Key words: Alzheimer’s disease, frontotemporal dementia, differential diagnosis, neuroimaging, neuropathology Słowa kluczowe: choroba Alzheimera, otępienie czołowo-skroniowe, diagnostyka różnicowa, neuroobrazowanie, neuropatologia Summary Organic dementia is dominated by primary degenerative disorders such as Alzheimer’s disease (AD) and frontotemporal dementia (FTD). FTD is a distinct clinical syndrome with behavioural, personality, emotional and language disturbances preceding the cognitive decline. This clinical presentation is distinctly different from that of AD which is characterized by early cognitive changes, such as memory impairment, aphasia and apraxia, and a relatively preserved personality and behaviour. The differences between these two conditions reflect the predominant topographic distribution of brain pathology. The differences in clinical profiles and treatment strategies will be highlighted. In both disorders loss of functional ability, development of behavioural disturbances and dependency impose heavy demands on family and other caregivers. This presentation will concentrate on early recognition and diagnosis, using systematic clinical evaluation, neuropsychological testing and different brain imaging methods. This is important for a successful development of therapeutic strategies for both cognitive and behavioural symptoms in FTD and AD. Background There is a growing interest in diagnosis of degenerative dementias other than Alzheimer’s disease (AD). This paper will present clinical characteristics in frontotemporal dementia (FTD) and AD and the possibility to differentiate between these two types of dementia. More than 100 years ago Arnold Pick [1] pointed out the association between lobar atrophy within the frontal and temporal lobes and symptoms such as personality change and dysphasia in dementia. The histopathological account for lobar atrophy was given by Alois Alzheimer in 1911 [2], one year before he succeeded Professor Karl Bonhoeffer in Wroclaw, Poland. Pure Pick’s disease is, however, rare and in the 1980ies the attention was drawn to another more prevalent type of dementia with cortical degeneration in frontal and temporal lobes. The first conference in Lund on „frontal lobe degeneration of non-Alzheimer type (FLD)” recognized the large number of dementia cases with frontal lobe degeneration, lacking the specific histopathology of PGP 24 Adres do korespondencji: Lars Gustafson Department of Psychogeriatrics University Hospital SE-221 85 Lund tel. +(46) (46) 177 454, fax +(46) (46) 177 457 [email protected] Copyright ©2004 Fundacja Ochrony Zdrowia Psychicznego 280 Lars Gustafson et al.: Frontotemporal dementia and Alzheimer’s disease Pick’s disease and AD [3, 4]. FLD and Pick’s disease have been diagnosed post mortem in 9% of 400 consecutive cases in a prospective longitudinal study of dementia [5]. So far we have included more than 150 FTD cases in the Lund Longitudinal Dementia Study and in about 50% it has been possible to obtain a post mortem examination. In 1994 the Lund and Manchester research groups published a consensus statement on clinical and pathological criteria for FTD [6]. The consensus presents the most prevalent and typical clinical features in the three neuropathological types of FTD: Pick’s disease, FLD and motor neuron disease with dementia (MNDD). The similarity and overlap between FTD, progressive aphasia and semantic dementia were dealt with in a consensus document on clinical criteria for frontotemporal lobar degeneration (FTLD) [7]. This paper will focus upon FTD as outlined in the 1994 Lund-Manchester consensus statement. AD is the most prevalent cause of severe dementia, responsible in its pure and mixed forms for at least 50% of all dementia conditions [5, 8]. Since the first publication in 1907 [9], AD has been described with increasing precision. The clinical picture in AD shows a number of symptoms and signs with more or less specific relationship to the distribution of the brain lesions. The majority of cases develop dysmnesia, dysphasia, dysgraphia, dysgnosia, dyspraxia and spatial disorientation in accordance with bilateral involvement of temporal limbic structures and the temporoparietal association cortex [10-12]. With increasing age at onset and longer duration there is a marked contribution of vascular components, especially white matter disease. This may aggravate and even add new features to the clinical picture in AD [13]. The etiology of FLD and its relationship to Pick’s disease are mainly unknown. In both diseases there is evidence for genetic factors with a positive family history in about half of the patients. In 1998 it was shown that the mutant gene causing disease in some of these families was the tau gene [14]. More than 25 different types of mutation have been identified in families with so called FTD and parkinsonism linked to chromosome 17 [15]. FTD has also been described in a large pedigree in Denmark with a linkage to a disease gene on chromosome 3 [16]. However, in the majority of FTD cases, so far no linkage to specific mutations or other etiological factors have been revealed. Pathological aspects The common neuropathology of FTD is bilateral frontotemporal degeneration. There are, however, certain histopathological differences between FLD, Pick’s disease and MNDD. FLD is characterized by a mild to moderate degeneration of mainly the frontal lobe and by a limited temporal lobe degeneration [3, 7, 17, 18], characterized by neuronal loss, microvacuolation and gliosis of the superficial cortical layers. These changes also involve the anterior but rarely the posterior cingulate gyrus, hippocampus and striate body. Frontal white matter often shows a mild to moderate loss of myelin [19]. There are no Alzheimer changes except mild ones compatible with age, no amyloidosis and no Pick bodies or inflated neurons in FLD. The cortical atrophy in Pick’s disease is severe, circumscribed with a knife-blade appearance, more often asymmetrical and many also involve striatum and hippocampus. The pathology in MNDD is similar to that of FLD but in addition there are ubiquitin-positive inclusions and spinal and bulbar motor system degeneration [20-22]. The degenerative pattern in AD is almost contrary to that of FTD originating in hippocampal and temporoparietal cortical regions and characterized by neuronal loss, neurofibrillary tangles and senile plaques. In a minority of AD cases there is also a marked degenerative involvement of frontal lobes [23]. Clinical features The following description of clinical manifestation in FTD and AD is based on post mortem verified cases in the Lund Longitudinal Dementia Study. The age at onset in FTD is usually between 35 and 70 years [4, 24, 25] but clinical onset may occur even earlier [26] and later. In our study the duration was 3 – 17 years (mean 8,1 ± 3,4 years) [27] which is in accordance with other studies [7, 18]. The clinical comparison is based on FTD and AD cases with clinical onset before 65 years of age. The clinical debut in both dementias is usually insidious with a slow and gradual progression although with individual variation. An abbreviated version of the clinical criteria in the Lund-Manchester consensus statement on FTD is presented in table 1. 281 Lars Gustafson et al.: Frontotemporal dementia and Alzheimer’s disease Table 1. „Clinical Diagnostic Features of frontotemporal dementia”. Consensus Statement by the Research Groups in Lund and Manchester (1994) Tab. 1. Cechy diagnostyczne otępienia czołowo-skroniowego. Stanowisko ekspertów naukowych z Lund i Manchester (1994) CORE DIAGNOSTIC FEATURES Behavioural Disorder Insidious onset and slow progression Early loss of personal and social awareness Early loss of insight Early signs of disinhibition Mental rigidity and inflexibility Stereotyped and perseverative behaviour Hyperorality Distractibility, impulsivity and impersistence Utilization behaviour Affective Symptoms Depression, anxiety, excessive sentimentality, suicidal ideation, delusion Hypochondriasis, bizarre somatic preoccupation Emotional unconcern, lack of empathy Amimia/aspontaneity Speech Disorder Progressive reduction of expressive speech Stereotypy of speech Echolalia, perseveration Late mutism Spatial Orientation and Praxis Preserved Physical signs Early primitive reflexes Early incontinence Late akinesia, rigidity and tremor Low and labile blood pressure Investigations Normal EEG despite clinically evident dementia Brain imaging (structural and/or functional): predominant frontal and/or anterior temporal abnormality Neuropsychology: profound failure on “frontal lobe” tests in the absence of severe amnesia, or perceptual spatial disorder SUPPORTIVE DIAGNOSTIC FEATURES Onset before 65 Positive family history of similar disorder in a first-degree relative Bulbar palsy, muscular weakness and wasting, fasciculations (motor neuron disease) 282 Lars Gustafson et al.: Frontotemporal dementia and Alzheimer’s disease Behavioural disorder The early stage of FTD is usually characterized by changes of personality and behaviour often with signs of disinhibition, loss of personal and social awareness and an alarming lack of insight into the present condition. Neglect of personal hygiene, restlessness, distractibility and mental inflexibility are common. As the disease progress disinhibition, lack of judgement and loss of insight may lead to impaired occupational performance, socially inappropriate behaviour, shop-lifting and traffic incidences. Changes in eating and oral habits such as overeating, food fads, excessive smoking and alcohol consumption and oral exploration of uneatable objects are common. Stereotyped ritualistic behaviour of an anacasticlike character is also observed. Echo phenomena and „utilization behaviour” [28] are also observed. Behavioural problems of this type are less common in AD. The habitual personality traits and social competence are usually better preserved in AD and under optimal conditions the AD patients appear more or less aware of their condition and their need of support. Affective symptoms Emotional changes are often noticed early in FTD and sometimes difficult to differentiate from non-organic mood disorder such as depression and hypomanic states. Many FTD patients have episodes of anxiety and depressed mood, sometimes with suicidal ideation and about 50% of our FTD cases had been treated with anti-depressants at an early stage of the disease. The affects in FTD are often characterized as bluntness, shallowness and indifference. Social withdrawal, loss of mimic movement and reduced speech are common in FTD and these symptoms may be misinterpreted as a major depression. Some patients become overactive and restless, sometimes showing inadequate smiling and laughing, while other patients are apathetic and inert. Irritability may be present, although hostility and acts of violence are relatively rare. In AD there is often an emotional change towards affective shallowness, but the habitual personality traits are better preserved. Anxiety and depressed mood may appear in AD especially at the early stage of the disease. Such emotional reactions are often related to the patient’s psychological conditions. Speech disorder FTD is characterized by a progressive reduction of expressive speech „Sprachverödung” [29] and “dissolution du langage” [30]. It often starts as a verbal aspontaniety with stereotyped comments and repetition of a limited number of phrases. The combination of palilalia, echolalia, late mutism and amimia (loss of spontaneous mimical movements), the so called PEMA-syndrome [31], is typical of FTD. In the end stage the patients may become mute but still capable of understanding verbal communication. The speech disorder in FTD has been described in similar ways by various research groups [7, 18, 32] and are different from the more global dysphasia with paraphasia and dysgraphia of post central type in late stages of AD. Logoclonia, a stuttering like phenomenon which is considered to be almost pathognominic of AD, is extremely uncommon in FTD [4]. Psychotic symptoms Psychotic features are prevalent in FTD as well as in AD [33-36]. In our longitudinal study hallucinations and delusions were reported in 20% of FTD and early onset AD, and in 50% of the late onset AD group [34]. The psychotic features in FTD are often bizarre [7] and badly controlled. The combination with signs of disinhibition, loss of insight, stereotype behaviour and language dysfunction may give the impression of functional psychosis, most often schizophrenia. The psychotic symptoms in early onset AD, however, seem more strongly related to cognitive failure and the degeneration of the temporoparietal association cortex. Thus the differences in psychotic symptoms between AD and FTD seem related to differences in the degenerative patterns [34, 35]. An association between psychosis and frontal lobe syndrome have, however, also been described in a subgroup of AD with marked frontal lobe involvement [34] and in patients with frontal ischemic white matter lesions [23, 37]. Physical signs Patients with FTD are remarkably free from somatic findings even in advanced stages. Neurological signs are generally limited to the presence of primitive reflexes, grasping, pouting and sucking, while extrapyramidal signs might appear later. Fasciculation, muscular wasting, dysarthria and dysphagia 283 Lars Gustafson et al.: Frontotemporal dementia and Alzheimer’s disease may develop in patients with FTD and indicate diagnosis of MNDD. Incontinence is an early symptom in a minority of FTD cases and more common in the middle and late stages of the disease. Low blood pressure and a tendency to orthostatic hypotension has been reported in 46% of FTD cases and 39% of AD cases [38]. Akinesia, rigidity and tremor may develop but are not as common as in AD. Increased muscular tension has been observed in 25% of FTD cases compared to 80% of the presenile AD cases. Epileptic seizures of grand mal type are found in a minority of FTD cases and in 50% of cases with AD [11, 39]. Endocrine functions in FTD are mainly normal, but thyroid hormone abnormalities are rather common in FTD (38%) compared to AD (9%) [15, 40]. Investigations Neuropsychology Changes of personality and behaviour are prominent features in FTD and tend to outweigh specific cognitive symptoms at an early stage of the dementing disease. Memory complaints are, however, often reported but also difficult to evaluate. The memory impairment may be mainly secondary to frontal regulatory disturbances causing inertia and lack of attention and planning. The patients usually remain oriented in place and time and can therefore negotiate their local environment without becoming lost. Relatives may remark that the patient wander several miles from home, yet finding his way back. With progress of the disease, impairment of memory, orientation and intellect become more obvious although not reaching the severity seen in AD. AD brings progress of mental deterioration dominated by dysmnesia, dysphasia, dyspraxia, dysgnosia and spatial disorientation. These symptoms have a strong relationship to the temporoparietal cortical involvement of the disease. Dysgraphia, dyscalculia, finger dysgnosia and right-left disorientation may sometimes develop a rather full-blown Gerstmann syndrome [11]. The increasing visuospatial dysfunction may affect recognition of objects and family members and even the patients own face in a mirror (mirror signs). In the early stage of FTD there may be very little abnormality revealed, even after thorough neuropsychological examination. However, impaired executive functions appear rather early and can affect cognition in tests measuring planning, selective attention and flexibility (the Trail Making Test, Stroop test and tests of Verbal fluency) [41]. With disease progression language test performance is impaired, speech output is reduced and there is frequently observed an impaired ability to understand abstract concepts and words (metaphorphical mening of proverbs) [42]. In varying degree episodic memory tests performance may be poor, possibly as a result of impaired attention and failure in effective strategies for retrieval. Visuospatial and perceptual functions are most often normal even late in the disease. A common observation from clinical practice is that FTD patients often show marked variation in the level of performance from one occasion to another. Repeated examinations are thus necessary for identifying the patient’s best level of performance. Obviously no highly standardised test administration is suitable. The predominant deficit in early AD is in the domain of episodic memory, on delayed tests of verbal as well as visuospatial recall, reflecting the hippocampal and posterior cinguli loci of the pathological changes [11, 42]. In contrast to FTD, there are deficits in visuospatial and perceptual functions in a relatively early stage. EEG EEG may often remain normal in the whole FTD group despite clinically evident dementia [43]. With a longer duration and increased severity some patients, however, develop moderately pathological EEG such as slowing. This is in contrast to patients with AD where EEG conventionally interpreted show a high percentage of abnormalities even at an early stage. Quantitative EEG analysis will probably improve the differential diagnosis between FTD, AD and vascular dementia (VaD) [43, 44]. Neuroimaging Structural and functional brain imaging have been recognized as extremely useful in the diagnosis and differential diagnosis of FTD, AD and other dementia. Progressive FTD is usually associated with cortical 284 Lars Gustafson et al.: Frontotemporal dementia and Alzheimer’s disease atrophy with more or less focal accentuation on CT or MR imaging. Although these findings are often non-conclusive with important overlap with findings in normals [45]. The anterior-posterior gradient of atrophic changes may, however, contribute to the differentiation from AD [46]. In a recent study 28 patients clinically diagnosed as FTD were compared to matched healthy controls [45]. Cortical atrophy predominantly in frontal regions was found in 70% of FTD patients compared to 22% in controls. The prevalence of white matter lesions, especially with anterior distributions was also higher in the FTD group compared to the controls. CT and MRI may in AD show a general atrophy, but a „normal” structural imaging does not exclude AD. Functional brain imaging measuring the regional cerebral blood flow (rCBF) and metabolism with SPECT, PET and other techniques show frontal and frontotemporal flow pathology in FTD with better preserved perfusion in posterior areas. rCBF measurement in AD show a marked contrast to this with consistent abnormalities in the temporoparietal areas [47-51]. These functional neuronal changes may at an early stage be mild and asymmetric in accordance with the clinical picture. Recently several diagnostic markers in cerebrospinal fluid (CSF) have been developed for early recognition and differential diagnosis of AD and FTD. This is important with respect to the possibility of pharmacological treatment available in AD. Three markers, total tau, phosphorylated tau and β-amyloid, are of special interest. FTD is characterized by normal to mild increase of total tau, normal phosphorylated tau and mild to moderately decrease of β-amyloid, while in AD often total tau and p-tau is moderately to markedly increased and β-amyloid show a moderate to marked decrease [52]. Differential diagnosis Patients with FTD are often misdiagnosed as having non-organic psychiatric conditions, especially at an early stage when non-cognitive symptoms prevail. The emotional changes can sometimes be difficult to differentiate from mood disorders, while behavioural disturbances might lead to the diagnosis of schizophrenia. Differential diagnosis of FTD on one hand and AD on the other is often possible using well-defined clinical diagnostic criteria, neuropsychological testing and brain imaging techniques. The Lund-Manchester Consensus of 1994, further developed in 1998 [53], is widely used as a guideline for clinical recognition of FTD [54]. In our clinical work we also use a combination of three diagnostic assessment scales for recognition of AD, FTD [5, 55] and vascular dementia (Ischemic Score [56]). Diagnoses based on the scoring profile in these three rating scales have been validated against rCBF findings, neuropathology, EEG and CSF findings [5]. Other diagnostic inventories for recognition of FTD have been developed by Kertesz et al [57] and Lebert et al [58]. Most guidelines for diagnosis of dementia such as NINCDS-ADRDA criteria for diagnosis of AD [59] may easily also include FLD and Pick’s disease. The revised third edition of the Diagnostic and Statistical Manual of mental disorders, DSM-III-R [60] presents Pick’s disease without further diagnostic guidelines, whereas DSM-IV [61] introduces Pick’s disease as „one of the pathologically distinct etiologies associated with frontotemporal atrophy”. The 10th edition of the International Classification of Diseases [62] describes „dementia in Pick’s disease” as slowly progressive dementia commencing in middle life with predominance of frontal lobe features and selective atrophy of the frontal and temporal lobes and without the pathological changes of AD. Thus the two major diagnostic guidelines describe dementia with frontotemporal lobe involvement, although not using the term FTD. Vascular brain damage, especially subcortical white matter disease and bilateral thalamic and caudate infarcts, may sometimes mimic FTD [23, 45], as many of these patients also display frontal lobe psychiatric features [63]. Differential diagnosis of FTD against Huntington’s disease and Creutzfeldt-Jakob’s disease may be critical when personality changes and psychotic features prevail while neurological characteristics are less obvious. 287 Lars Gustafson et al.: Frontotemporal dementia and Alzheimer’s disease Treatment and care After establishing the clinical diagnosis of FTD it is of central importance to follow up and support the patient and the family involved. Many questions will arise and difficult decisions have to be made and therefore it is essential to explain the nature of the patients changed behaviour and current problems. In this context it is important not to forget the children who may still be at school age or on their way to establish an independent life. It may be possible for the patient to continue professional work for a limited time. However, it is often in the field of work, especially in social interaction, that the earliest signs of the brain disease emerge. When the patient can no longer be taken care of at home, alternative optimal placements must be arranged. This care may sometimes be difficult because of the patients disturbing symptoms and lack of insight. Temporary admissions may make it possible for the family to cope with the emotional and practical problems. Access to trained staff strongly increases the understanding and management of the patient including achievement of a safe environment. Many patients have an urgent need to walk long distances. Utilization behaviour and tendency to overeat and put uneatable items in their mouth have to be met with active strategies and diversion and less by restrictions and pharmacological sedation. The inappropriate behaviour may, however, be difficult to deal with. Relatives and carers need continuous support and advice, knowing the behaviour is a consequence of a brain disease. When the patient has lost the ability to communicate verbally it is most important to remember that the ability to observe and understand may still remain. The spouse and family members often report social isolation and loneliness. The Alzheimer Associations may offer these people information and support in various forms. The patient and the family are often comparatively young and knowing that many others are suffering from this type of disease and that medical research is also focusing upon FTD might offer some comfort. The experience of FTD has encouraged our department to develop a National Competence Centre [64], supported by the Ministry of Health and Social Affairs in Sweden. By this project we can contribute with advice to medical staff, handling FTD patients and carers, and develop programmes for training and support to the staff and the families. Currently, no medication is thought to prevent, reverse or even slow the progress of FTD. The cholinergic drugs that delay the progression of AD for some patients are not likely to help FTD patients, who do not have a cholinergic deficit [65]. Serotonin-boosting antidepressants may be effective in treating some behavioural disturbances [66]. Antipsychotic medications may alleviate symptoms in FTD patients suffering from delusions and hallucinations. However, our clinical experience is that these medications sometimes seem to exacerbate FTD symptoms. Every drug has side effects but it may sometimes be used to the patient’s advantage. Still, pharmacological treatment goes only so far, and taking care of patients with FTD presents a huge challenge. Treatment and care of patients with AD have to consider the natural course of the disease, the individual variation, comorbidity with other disease, and the contribution of somatic and psychiatric complications. The introduction of pharmacological treatment in AD has changed the attitudes with a more optimistic and active approach to rehabilitation and care. The increasing need for alternative forms of care such as group-living and psychiatric day hospital for those many patients, who whish to remain in their home [67, 68], is obvious. Conclusion FTD is a group of dementias with a common pattern of cortical and clinical pathology and increasing evidence of underlying genetic factors. From the clinical point of view, FTD stands out as a diagnostic and therapeutic challenge. The early diagnosis is a prerequisite for adequate treatment and care. The long duration of the disease has a strong impact on the family, that needs professional support through the many difficult years. The clinical picture in FTD shows a wide spectrum of mental and behavioural disorders with clinical similarity to schizophrenia, mood disorder, obsessive-compulsive disorder, and 288 Lars Gustafson et al.: Frontotemporal dementia and Alzheimer’s disease sociopathic behaviour, probably strongly related to the involvement of prefrontal, anterior temporal, and anterior cingulate cortex. Many clinical and experimental studies [69] show the central role of the anterior cingulate gyrus in the executive system involved in emotions and cognition, social interaction, vocalization, mimical movements, and responses to pain, and our observations strongly support such ideas. The clinical dissimilarity between FTD and AD is in accordance with the almost contrary topographic degenerative patterns in these two major degenerative dementias. This is also valid for the cingulate gyrus, its anterior part being involved in FTD and its posterior part in AD. The importance of the frontal association cortex and its connections to anterior limbic structures in „functional” mental diseases such as schizophrenia [70, 71] has to be considered in the search for etiology and therapeutic strategies in FTD. Acknowledgement This study was supported by the Swedish Research Council, project 3950, Alzheimer Foundation Sweden and the Sjöbring Foundation. The authors wish to thank Dr Elisabet Englund, Department of Pathology, Lund University, for valuable assistance regarding the neuropathology, Professor Ingmar Rosén, Department of Clinical Neuroscience, Lund University, regarding SPECT-imaging and Dr Elna-Marie Larsson, Department of Diagnostic Radiology, Lund University regarding the MRI. References [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] Pick A. Über die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Med Wochenschr 1892; 17: 165-167. Alzheimer A. Über eigenartige Krankheitsfälle der späteren Alters. Zeitschrift für die Gesamte Neurologie und Psychiatrie 1911; 4: 356-385. Brun A. Frontal lobe degeneration of non-Alzheimer type. I. Neuropathology. Arch Gerontol Geriatr 1987; 6(3): 193-208. Gustafson L. Frontal lobe degeneration of non-Alzheimer type. II. Clinical picture and differential diagnosis. Arch Gerontol Geriatr 1987; 6(3): 209-223. Brun A, Gustafson L. I. The Lund Longitudinal Dementia Study: A 25-year perspective on neuropathology, differential diagnosis and treatment. In: Corain B, Iqbal K, Nicolini M, Winblad B, Wisniewski H, Zatta P, editors. Alzheimer’s disease: Advances in clinical and basic research: John Wiley & Sons Ltd, 1993. pp. 4-18. Brun A, Englund E, Gustafson L, Passant U, Mann DMA, Neary D, Snowden JS. Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry 1994; 57(4): 416-418. Neary D, Snowden JS, Northen B, Goulding P. Dementia of frontal lobe type. J Neurol Neurosurg Psychiatry 1988; 51(3): 353-361. Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci 1970; 11(3): 205-242. Alzheimer A. Über eine eigenartige erkankung der hirnrinde. Allgemeine Zeitschrift für Psychiatrie und psychisch-gerichtliche Medizin, Berlin 1907; 64: 146-148. Sjögren T, Sjögren H, Lindgren AG. Morbus Alzheimer and morbus Pick; a genetic, clinical and patho-anatomical study. Acta Psychiatr Neurol Scand 1952; 82: 1-152. Brun A, Gustafson L. Distribution of cerebral degeneration in Alzheimer’s disease. A clinico-pathological study. Arch Psychiatr Nervenkr 1976; 223(1): 15-33. Lauter H. Über Spätformen der Alzheimerschen Krankheit und ihre Beziehung zur senilen Demenz. Psychiatr Clin (Basel) 1970; 3(3): 169-189. Englund E, Brun A, Gustafson L. A white matter disease - dementia of Alzheimer’s type. Clinical and morphological correlates. International Journal of Geriatric Psychiatry 1989; 4: 87-102. Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, 289 Lars Gustafson et al.: Frontotemporal dementia and Alzheimer’s disease [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] [31] [32] [33] [34] [35] [36] [37] [38] Kwon JM, Nowotny P, Heutink P, et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998; 393(6686): 702-705. Rosso SM, Landweer EJ, Houterman M, Donker Kaat L, van Duijn CM, van Swieten JC. Medical and environmental risk factors for sporadic frontotemporal dementia: a retrospective case-control study. J Neurol Neurosurg Psychiatry 2003; 74(11): 1574-1576. Brown J, Ashworth A, Gydesen S, Sorensen A, Rossor M, Hardy J, Collinge J. Familial non-specific dementia maps to chromosome 3. Hum Mol Genet 1995; 4(9): 1625-1628. Brun A. Frontal lobe degeneration of non-Alzheimer type revisited. Dementia 1993; 4(3-4): 126-131. Knopman DS, Mastri AR, Frey WH, 2nd, Sung JH, Rustan T. Dementia lacking distinctive histologic features: a common non-Alzheimer degenerative dementia. Neurology 1990; 40(2): 251-256. Englund E, Brun A. Frontal lobe degeneration of non-Alzheimer type. IV. White matter changes. Arch Gerontol Geriatr 1987; 6(3): 235-243. Neary D, Snowden JS, Mann DM, Northen B, Goulding PJ, Macdermott N. Frontal lobe dementia and motor neuron disease. J Neurol Neurosurg Psychiatry 1990; 53(1): 23-32. Mitsuyama Y. Presenile dementia with motor neuron disease. Dementia 1993; 4(3-4): 137-142. Mitsuyama Y. Dementia with motor neuron disease. Neuropathology 2000; 20 Suppl: S79-81. Brun A, Gustafson L. Psychopathology and frontal lobe involvement in organic dementia. In: Iqbal K, D.R.C. M, Winblad B, Wisnewski HM, editors. Alzheimer’s disease: Basic Mechanisms, diagnosis and therapeutic strategies. London: John Wiley & Sons, 1991. pp. 27-33. Neary D. Dementia of frontal lobe type. J Am Geriatr Soc 1990; 38(1): 71-72. Pasquier F, Lebert F, Lavenu I, Guillaume B. The clinical picture of frontotemporal dementia: diagnosis and follow-up. Dement Geriatr Cogn Disord 1999; 10 Suppl 1: 10-14. Willert C, Spitzer C, Herzer R, Kirsch G. [Early manifestation of fronto-temporal dementia]. Nervenarzt 2000; 71(1): 44-49. Gustafson L. Clinical picture of frontal lobe degeneration of non-Alzheimer type. Dementia 1993; 4(3-4): 143-148. Lhermitte F, Pillon B, Serdaru M. Human autonomy and the frontal lobes. Part I: Imitation and utilization behavior: a neuropsychological study of 75 patients. Ann Neurol 1986; 19(4): 326-334. Schneider C. Über Picksche Krankheit. Monatschr Psychiat Neurol 1927; 65: 230-275. Delay J, Neveu P, Desclaux P. Les dissolutions du langage dans la maladie de Pick. Revue Neurologique 1944; 76: 37-38. Guiraud P. Psychiatrie clinique. Paris: Le Francois, 1956. Snowden JS, Neary D. Progressive language dysfunction and lobar atrophy. Dementia 1993; 4(3-4): 226-231. Burns A, Jacoby R, Levy R. Psychiatric phenomena in Alzheimer’s disease. I: Disorders of thought content. Br J Psychiatry 1990; 157: 72-76, 92-74. Gustafson L, Risberg J. Deceptions and delusions in Alzheimer’s disease and frontal lobe dementia. In: Katona C, Lewy R, editors. Delusions and hallucinations in old age. London: Gaskell, 1992. pp. 218-229. Lopez OL, Gonzales MP, Becker JT, Reynolds CF, Sudilovsky A, DeKosky ST. Symptoms of depression of psychosis in Alzheimer’s disease and frontotemporal dementia. Neuropsychiatry, Neuropsychology, and Behavioral Neurology 1996; 9: 151-161. van Mansfelt J. Pick’s disease. A syndrome of lobar, cerebral atrophy; its clinico-anatomical and histopathological types. Thesis. Enschede, Utrecht, 1954. Miller BL, Cummings JL, Villanueva-Meyer J, Boone K, Mehringer CM, Lesser IM, Mena I. Frontal lobe degeneration: clinical, neuropsychological, and SPECT characteristics. Neurology 1991; 41(9): 1374-1382. Passant U, Warkentin S, Gustafson L. Orthostatic hypotension and low blood pressure in organic dementia: a study of prevalence and related clinical characteristics. Int J Geriatr Psychiatry 1997; 12(3): 395-403. 290 Lars Gustafson et al.: Frontotemporal dementia and Alzheimer’s disease [39] [40] [41] [42] [43] [44] [45] [46] [47] [48] [49] [50] [51] [52] [53] [54] [55] [56] [57] [58] [59] [60] [61] Lauter H. Zur Klinik und Psychopatologie der Alzheimerschen Krankheit. Psychiatr Clin (Basel) 1968; 1(2): 85-108. Fäldt R, Passant U, Nilsson K, Wattmo C, Gustafson L. Prevalence of thyroid hormone abnormalities in elderly patients with symptoms of organic brain disease. Aging (Milano) 1996; 8(5): 347-353. Gregory CA, Serra-Mestres J, Hodges JR. Early diagnosis of the frontal variant of frontotemporal dementia: how sensitive are standard neuroimaging and neuropsychologic tests? Neuropsychiatry Neuropsychol Behav Neurol 1999; 12(2): 128-135. Elfgren C, Brun A, Gustafson L, Johanson A, Minthon L, Passant U, Risberg J. Neuropsychological tests as discriminations between dementia of Alzheimer type and frontotemporal dementia. International Journal of Geriatric Psychiatry 1994; 9: 635-642. Rosen I, Gustafson L, Risberg J. Multichannel EEG frequency analysis and somatosensory-evoked potentials in patients with different types of organic dementia. Dementia 1993; 4(1): 43-49. Johannesson G, Brun A, Gustafson I, Ingvar DH. EEG in presenile dementia related to cerebral blood flow and autopsy findings. Acta Neurol Scand 1977; 56(2): 89-103. Larsson E, Passant U, Sundgren PC, Englund E, Brun A, Lindgren A, Gustafson L. Magnetic resonance imaging and histopathology in dementia, clinically of frontotemporal type. Dement Geriatr Cogn Disord 2000; 11(3): 123-134. Förstl H, Besthorn C, Hentschel F, Geiger-Kabisch C, Sattel H, Schreiter-Gasser U. Frontal lobe degeneration and Alzheimer’s disease: a controlled study on clinical findings, volumetric brain changes and quantitative electroencephalography data. Dementia 1996; 7(1): 27-34. Risberg J, Gustafson L. Regional cerebral blood flow measurements in the clinical evaluation of demented patients. Dement Geriatr Cogn Disord 1997; 8(2): 92-97. Jagust WJ, Reed BR, Seab JP, Kramer JH, Budinger TF. Clinical-physiologic correlates of Alzheimer’s disease and frontal lobe dementia. Am J Physiol Imaging 1989; 4(3): 89-96. Miller BL, Itti L, Li J, et al. Atrophy-corrected cerebral blood flow in frontotemporal dementia. Facts and Research in Gerontology 1995: 93-103. Ingvar DH. History of brain imaging in psychiatry. Dement Geriatr Cogn Disord 1997; 8(2): 66-72. Passant U, Elfgren C, Risberg J, Rosén I, Gustafson L. Brain imaging in frontotemporal dementia. International Journal of Geriatric Psychopharmacology 2000; 2: 90-96. Blennow K, Vanmechelen E, Hampel H. CSF total tau, Abeta42 and phosphorylated tau protein as biomarkers for Alzheimer’s disease. Mol Neurobiol 2001; 24(1-3): 87-97. Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51(6): 1546-1554. Gregory CA, Hodges JR. Frontotemporal dementia: Use of consensus criteria and prevalence of psychiatric features. Neuropsychiatry Neuropsychol Behav Neurol 1996; 9(145-153). Gustafson L, Nilsson L. Differential diagnosis of presenile dementia on clinical grounds. Acta Psychiatr Scand 1982; 65(3): 194-209. Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, McAllister VL, Marshall J, Russell RW, Symon L. Cerebral blood flow in dementia. Arch Neurol 1975; 32(9): 632-637. Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc 2000; 6(4): 460-468. Lebert F, Pasquier F, Souliez L, Petit H. Frontotemporal behavioral scale. Alzheimer Dis Assoc Disord 1998; 12(4): 335-339. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34(7): 939-944. American Psychiatric Association. DSM-III-R, Diagnostic and statistical manual of mental disorders. Washington DC: APA, 1987. American Psychiatric Association. DSM-IV, Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Association, 1994. 291 Lars Gustafson et al.: Frontotemporal dementia and Alzheimer’s disease [62] [63] [64] [65] [66] [67] [68] [69] [70] [71] World Health Organization. ICD-10, World Health Organization Tenth Revision of the International Classification of Diseases. Geneva: WHO, 1992. Gustafson L, Passant U. Clinical pathological correlates. In: O’Brien J, Ames D, Gustafson L, Folstein M, Chiu E, editors. Cerebrovascular disease, cognitive impairment and dementia. London: Martin Dunitz, 2004. pp. 197-210. Passant U. The Swedish National Competence Centre for FTD - a model for supporting facilities. Dement Geriatri Cogn Disord 2004; 17: 378. Procter AW, Qurne M, Francis PT. Neurochemical features of frontotemporal dementia. Dement Geriatr Cogn Disord 1999; 10 Suppl 1: 80-84. Lebert F, Stekke W, Hasenbroekx C, Pasquier F. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord 2004; 17(4): 355-359. Annerstedt L, Sanada J, Gustafson L. A dynamic long-term care system for the demented elderly. Int Psychogeriatr 1996; 8(4): 561-574. Johansson A, Gustafson L. Psychiatric symptoms in patients with dementia treated in a psychogeriatric day hospital. Int Psychogeriatr 1996; 8(4): 645-658. Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain 1995; 118 (Pt 1): 279-306. Ingvar DH, Franzen G. Abnormalities of cerebral blood flow distribution in patients with chronic schizophrenia. Acta Psychiatr Scand 1974; 50(4): 425-462. Sabri O, Erkwoh R, Schreckenberger M, Owega A, Sass H, Buell U. Correlation of positive symptoms exclusively to hyperperfusion or hypoperfusion of cerebral cortex in never-treated schizophrenics. Lancet 1997; 349(9067): 1735-1739. Otrzymano/Received 04.10.2004 Zatwierdzono do druku/Accepted 25.11.2004 292 Lars Gustafson et al.: Frontotemporal dementia and Alzheimer’s disease KOMUNIKAT REDAKCJI Tłumaczenia artykułów publikowanych języku angielskim są dostępne na naszej stronie internetowej w dziale PGP ON-LINE www.fozp.org.pl