* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ACID-BASE AND ELECTROLYTE TEACHING CASE Beer
Survey
Document related concepts
Transcript
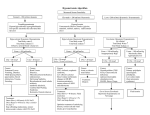
ACID-BASE AND ELECTROLYTE TEACHING CASE Beer Potomania: An Unusual Cause of Hyponatremia at High Risk of Complications From Rapid Correction Shalin R. Sanghvi, MD,1,2 Paul S. Kellerman, MD,1,2 and Lisa Nanovic, DO1,2 INDEX WORDS: Beer; potomania; hyponatremia; management; algorithm; treatment; complications. CASE REPORT An unusual syndrome of hyponatremia was described in beer drinkers dating back to the early 1970s. The syndrome “beer potomania” is used to describe a patient who presents with hyponatremia in conjunction with low daily solute intake and excessive beer drinking. This pathophysiological state results in patients at serious risk of rapid correction of hyponatremia and its neurological sequelae. This article reviews the literature for both beer potomania and osmotic demyelination syndrome (ODS). Two patients with beer potomania with differing outcomes are presented, with crucial differences in management. The article describes pathophysiological characteristics and the unique management challenges of beer potomania. Last, recommendations and an algorithm for the treatment of patients with hyponatremia caused by beer potomania are presented as a potential guide to prevent the catastrophic outcome of ODS. Patient 1 Clinical History A 39-year-old woman was admitted to the hospital after being brought by a family member because of a 24-hour history of increasing confusion. Her medical history was remarkable for long-standing alcohol use and an alcohol withdrawal seizure approximately 2 years before this presentation. Her daily intake was approximately 18 cans of beer. Recent history included antibiotic use for a tooth infection and diarrhea after starting the antibiotics. The family member was unaware if there was recent binge drinking. The patient’s initial mental status showed a somnolent woman with mildly slurred speech and oriented to person, place, and time, but with difficulty with short-term memory. Serum sodium level on presentation was 100 mEq/L (mmol/L; Table 1). Additional Investigations hours after ED presentation. On the general medical floor, she had urinary output of 500 mL/h, and serum sodium level increased by 6 mEq/L (mmol/L) over 4 hours (Fig 1). Dextrose 5% in water (D5W) was started at a rate to match urine output to prevent a further increase in serum sodium level. Because of excessive nursing needs for serial laboratory draws, hourly recordings of urinary output, and titration of IV fluids, the patient was transferred to the intensive care unit 12 hours after ED presentation. The first urine osmolality measured 4 hours after ED presentation was 218 mOsm/L; however, rechecks at 12 and 18 hours after presentation showed values of 50 and 48 mOsm/L, respectively. The patient was treated with IV clindamycin for empiric antibiotic coverage. Review of the patient’s records during the first 24 hours showed a total of 8,500 mL of IV fluids (NS solution accounted for 1,200 mL) and 7,600 mL of urine output. In the subsequent 24 hours, the patient received 4,300 mL of D5W and had 4,300 mL of urine output. Serum sodium level increased 15 mEq/L (mmol/L) during the first 24 hours and 24 mEq/L (mmol/L) during the first 48 hours (Fig 1). On day 7, her level of alertness started to decrease, and on day 9, magnetic resonance imaging of the brain showed findings consistent with pontine and extrapontine myelinolysis. Patient 2 Clinical History A 63-year-old man with a history of alcoholism presented to the ED with weakness and dizziness after a 2-week history of binge drinking. The patient had been drinking approximately 15 to 20 cans of beer daily and had very little food intake. On examination, the patient was oriented to person, place, and time, with mild difficulty following commands. Serum sodium level at the time of presentation was 104 mEq/L (mmol/L; Table 1). The patient had no laboratory or clinical evidence of cirrhosis or congestive heart failure. Computed tomographic scan of the head on admission had normal findings. Diagnosis Symptomatic severe hyponatremia was diagnosed, possibly caused by beer potomania. Clinical Follow-Up In the emergency department (ED), the patient was administered 1 g of intravenous (IV) cefazolin and 1 L of normal saline (NS) solution containing magnesium and thiamine. The patient was transferred to a general medicine bed 5 From the 1University of Wisconsin, Madison; and 2Veterans Affair Hospital, Middleton, WI. Received March 26, 2007. Accepted in revised form July 12, 2007. Address correspondence to Shalin R. Sanghvi, MD, 3034 Fish Hatchery Rd, Fitchburg, WI 53713. E-mail: [email protected] © 2007 by the National Kidney Foundation, Inc. 0272-6386/07/5004-0019$32.00/0 doi:10.1053/j.ajkd.2007.07.015 American Journal of Kidney Diseases, Vol 50, No 4 (October), 2007: pp 673-680 673 674 Table 1. Summary of Beer Potomania Cases Presentation Laboratory Results Author (year) Demanet (71) Patients no. Neurological Symptoms on Presentation Na (mEq/L) K (mEq/L) BUN (mg/dL) U osm (mosm/L) U Na (mos m/L) Treatment Serum Na Change 1 2 Unconscious, seizure Weakness 107 105 2.6 1.3 12 15 N/A N/A N/A N/A N/A N/A N/A N/A 3 4 Weakness Unconscious 104 103 2.6 2.5 30 13 N/A N/A N/A N/A N/A 10 mmol/48 h Gwinup (72) 5 6 7 8 Weakness, “grand mal” Unconscious, “grand mal” Unconscious, “grand mal” Weakness 101 99 98 122 1.4 1.8 4.4 5.1 19 18 30 N/A N/A N/A N/A N/A N/A N/A N/A N/A N/A 300 MEQ Na IV d 1 and 2 N/A N/A N/A Fluid restriction N/A N/A N/A 20 mmol/72 h Hilden (75) 9 123 2.7 N/A N/A N/A 0.9% NS 2–3 L 12 mmol/48 h 109 2.5 N/A 79 N/A No IVFs 15 mmol/48 h 108 2.7 N/A 69 N/A 0.9% NS 2–3 L 19 mmol/48 h 127 2.5 N/A N/A N/A No IVFs 2 mmol/48 h 117 3.1 N/A N/A N/A No IVFs 9 mmol/48 h N/A 199 5.6 Fluid restriction 8 mmol/48 h N/A N/A 9 mmol/48 h N/A N/A N/A 1.8% NS ⫻ 6 h then 10 DW or 0.9% NS 3% NS ⫻ 30 mL, 0.9% NS @ 300 ml/h 0.9% NS @ 100 mL/h 0.9% NS @ 150 mL/h and 3% NS @ 20 mL/ h ⫻ 160 Ml NS 3 L/24 h 0.45 NS ⫹ KCl supplements NS, later fluid restriction with D5 Swenson (76) 14 Debility, dizziness, confusion Debility, dizziness, confusion Debility, dizziness, confusion Debility, dizziness, confusion Debility, dizziness, confusion Weakness 106 3.8 Evans (85) 15 Confused, restless 118 4.1 Joyce (86) 16 110 3 Fenves (95) 17 18 Agitation, confusion, seizures Tremor Unconscious, seizures 121 97 3.6 3.6 4 2 50 338 1 12 Kelly (98) Lens (01) 19 20 Unconscious Confusion/weakness 109 97 3.6 2.1 16 14 340 N/A ⬍10 N/A Sanghvi (06) 21 Weakness 100 2.7 4 218 53 22 Weakness 104 4.3 7 547 10 10 11 12 13 2.3 Fluid restriction 14 mmol/24 h Outcome N/A Death - brain autopsy with atrophy of mammillary bodies Death - no brain autopsy N/A Death - normal brain autopsy Death - brain autopsy with CPM N/A No neurological sequelae reported No neurological sequelae reported No neurological sequelae reported No neurological sequelae reported No neurological sequelae reported No neurological sequelae reported No apparent neurological sequelae Long-term impaired memory, confabulation Discharged d 2, lost to follow-up 13 mmol/13 h 30 mmol/48 h Not reported No apparent neurological sequelae 20 mmol/48 h 21 mmol/48 h ODS ODS 15 mmol/24 h, 24 mmol/ 48 h 7 mmol/24 h, 14 mmol/48 h ODS No apparent neurological sequelae Sanghvi et al Note: To convert BUN in mg/dL to mmol/L, multiply by 0.357; sodium and potassium in mEq/L and mmol/L are equivalent. Abbreviations: Na, sodium; K, potassium; BUN, blood urea nitrogen; U osm, urine osmolality; U Na, urine sodium; N/A, not available; IV, intravenous; CPM, central pontine myelinolysis; NS, normal saline solution; IVF, intravenous fluid; KCl, potassium chloride; ODS, osmotic demyelination syndrome. Risks and Management of Beer Potomania 675 Figure 1. Time graph for patients 1 and 2. Note: Sodium (Na) in mEq/L and mmol/L is equivalent. Abbreviations: ER, emergency room; ICU, intensive care unit; UO, urine output; NS, normal saline; D5W, dextrose 5% in water. Additional Investigations The patient was not being administered antibiotics and had no laboratory or clinical evidence of cirrhosis or congestive heart failure. quent urine osmolality was 71 mOsm/L (6 hours after ED presentation). The 24-hour increase in serum sodium level was 7 mEq/L (mmol/L), and the 48-hour increase was 14 mEq/L (mmol/L). The patient was discharged on hospital day 6 without clinical evidence of neurological sequelae. Diagnosis DISCUSSION Severe hyponatremia was diagnosed, possibly caused by beer potomania. Background Clinical Follow-Up Based on the tentative diagnosis of beer potomania and mild neurological symptoms, the patient’s fluid intake was restricted to 1 L. The patient’s only IV fluids were a dose of moxifloxacin (in 0.9% NS solution) in the ED. After transfer to the intensive care unit 2 hours after ED presentation for further monitoring, the patient had an impressive diuresis of 3,000 mL during the subsequent 2 hours. Serum sodium level increased 7 mEq/L (mmol/L) during the first 9 hours (Fig 1). D5W was started at a rate to match urine output. Diuresis decreased to approximately 150 mL/h. Total urine output during the first 24 hours was 6,500 mL, and total IV fluids were 4,500 mL (NS solution accounted for 250 mL). Review of the patient’s laboratory test results showed urine osmolality at presentation of 547 mOsm/L; however, subse- Cases of a hypo-osmolality syndrome in beer drinkers were first described by Gwinup et al1 in 1972, and series of patients with similar presentations were described by Hilden and Svendsen2 in 1975 and Swenson and Rater3 in 1976. In earlier cases, the cause of hyponatremia was complicated by confounding factors, including diuretic use and lack of evaluation for such diseases as congestive heart failure or cirrhosis. However, with repeated documented cases of patients presenting with hyponatremia in conjunction with excessive beer drinking and low daily solute 676 intake, it is evident that there is a syndrome of hyponatremia virtually unique in beer drinkers. Electrolyte abnormalities are not uncommon in patients admitted with a history of alcohol use. In the study by Liamis et al,4 17% of chronic alcoholic patients had hyponatremia. Review of internal data from the University of Wisconsin and Veterans Affairs Hospital in Middleton, WI, showed hyponatremia in greater than 5% of patients admitted with alcoholism as a coding diagnosis. In addition to beer potomania, there are a number of other potential causes of hyponatremia in alcoholics, including cirrhosis, syndrome of inappropriate antidiuretic hormone (ADH), congestive heart failure, hypovolemia, and pseudohyponatremia secondary to dyslipidemia.4 Laboratory values for 22 published cases, including the 2 mentioned here, were reviewed to identify features specific to beer potomania.1-3,5-11 Of the laboratory data available, consistent findings among patients included severe hyponatremia (mean serum sodium, 108 mEq/L [mmol/L]), hypokalemia (mean serum potassium, 3 mEq/L [mmol/L]), mild neurological symptoms on presentation (typically confusion), low blood urea nitrogen level, brisk diuresis in response to solute intake, and low urine sodium level. Although not consistently reported in patients with beer potomania, low urine osmolality on admission laboratory test results was not a consistent finding (Table 1). Many similarities were noted in the presentation of patients with beer potomania. In addition to the history of excess beer drinking, often a recent history of binge drinking or illness was present. This may potentially precipitate a rapid decrease in serum sodium levels, which causes the patient to acutely present with such clinical symptoms of hyponatremia as confusion, altered mental status, and gait disturbances. The relationship between neurological lesions and rapid correction of chronic hyponatremia was noted in the late 1970s, and a direct relationship was established in the early 1980s.12,13 Specific neurological findings included central and extrapontine myelinolysis, now known as ODS. ODS is diagnosed by means of magnetic resonance image, with findings of hyperintense lesions on T2-weighted images. Most signal changes are located in the central part of the pons, medulla oblongata, and mesencephalon.14 Clinical features include upper motor neuron signs, pseudobulbar palsy, spastic quadriparesis, Sanghvi et al and mental disorders ranging from mild confusion to coma. Two retrospective reviews by Sterns et al15 and Ellis16 reported neurological sequelae in some patients when the change in serum sodium during a 24-hour period was greater than 12 mEq/L (mmol/L) and 10 mEq/L (mmol/L), respectively. Case reports describe ODS in patients with a rate of correction less than 10 mEq/L (mmol/L) per 24 hours.9 Our literature review found 22 cases (including ours) of beer potomania. In these cases, 8 patients had complications (36%): 4 of the 8 had ODS (18%), whereas the other 4 died (18%). Management varied from hypertonic saline solution for brief periods to free-water restriction and even free-water supplementation in certain cases. Brisk diuresis was common in many patients after admission (Table 1). Understanding the pathophysiological state is pivotal to recognizing the possibility of rapid correction and the possible complications. Furthermore, because ODS may not present until 2 to 3 days after the change in serum sodium levels, it is important to delineate management goals at the initial evaluation. Pathophysiology Hyponatremia in beer drinkers occurs because of water intake that exceeds excretory capacity (described next), modified by the possibility of inappropriate ADH secretion on admission. Our review of the 22 cases shows that hyposthenuria on admission was not a consistent finding (although urine sodium levels were low). Recognizing this finding prevents the erroneous dismissal of beer potomania. In the majority of cases, after admission, brisk water diuresis ensued, leading to the likelihood that when infections and volume status were treated, the patient’s ADH secretion decreased to a level commensurate with the degree of hypo-osmolality. Free-water clearance is dependant on solute excretion and urinary diluting capability.17 Based on a normal diet, typical osmole excretion is approximately 600 to 900 mOsm/d. This osmolar load is caused by urea generation from protein (10 g of protein produces ⬃50 mOsm of urea), in addition to dietary sodium and potassium intake. If the maximum urinary dilution capability is 50 mOsm/L, a large amount of water (⬎20 L) must be ingested under normal situations to overwhelm the capacity for urinary dilution, as seen Risks and Management of Beer Potomania in psychogenic polydipsia. If solute excretion decreases, the ability to excrete free water becomes limited. For example, if the patient excretes only 100 mOsm/d, greater than 2 L of fluid intake with a urinary dilution capability of 50 mOsm/L will result in net water retention and subsequently hyponatremia. Patients with beer potomania have a history of significant beer drinking, often long term, in conjunction with a poor diet. The net result is very low osmole intake because beer has very little sodium and no protein, but has some calories that prevent endogenous protein breakdown (urea generation). Because the obligatory solute loss in a day is approximately 250 mOsm in these patients,7 with a urinary dilution capability of 50 mOsm/L, water intake greater than 5 L (or 14 cans of beer) results in hyponatremia. The net effect is an excess of solvent (electrolyte-free water) without the solute for diuresis. ADH levels are expected to be suppressed in patients with beer potomania.4 The low ADH levels limit free-water reuptake in the collecting tubules of the kidney and explain why these patients have brisk diuresis when solute is presented. Sodium chloride in IV fluids is a common source of the solute load while hospitalized. Less obvious forms include “banana bag” (NS solution with thiamine, magnesium, and multivitamin), antibiotics, and food. Urine osmolality on recheck after the solute is introduced is low in these patients because of the low ADH levels. Based on a solute concentration of 308 mEq/L (mmol/L) in 0.9% sodium chloride solution and the kidney’s diluting ability of 50 mOsm/L, significant diuresis can occur with 1 L of NS solution in the setting of a low-ADH state. This water diuresis can produce large increases in serum sodium levels in a short period. Attempting to replace this with electrolyte-free water to prevent a rapid increase in sodium levels can be difficult. In patients with hyponatremia, cellular swelling occurs because the fluid shifts from the hypotonic extracellular space to the relative hypertonic intracellular space. Because of the confined space of the brain, this swelling can lead to deleterious results, including cerebral herniation. Cerebral adaptation occurs in response to decreased intracellular tonicity. There is an acute response consisting of electrolyte shifts of mainly 677 sodium and potassium out of the cell, and a chronic response characterized by loss of intracellular organic osmoles because of the persistent osmotic imbalance.18 Because organic osmoles take 5 to 7 days to return to normal levels, the long-term adaptation is the major concern when correcting hyponatremia. Oligodendrocytes are particularly sensitive to the osmotic stress that occurs with correction at a rate that the intracellular osmolality remains hypotonic relative to the extracellular environment. Although the exact mechanism is not known, this stress leads to both pontine and extrapontine myelinolysis, known as ODS.19 Symptoms can be related to damage of the corticospinal or corticobulbar tracts within the basis pontis or secondary to extrapontine involvement and manifest as ataxia, movement disorders, or irregular behavior. Often the symptoms of ODS present as a biphasic course, with improvement in the initial symptoms of hyponatremia and a secondary neurological decline.8 However, they also can be in a monophasic course, making the diagnosis of ODS very difficult.19 Because of the underlying pathophysiological state and risk of ODS, challenging questions at the time of initial evaluation include whether IV fluid is needed and, if so, what type and how much IV fluid is needed, and last, what the treatment goals are. Management Although most patients with hyponatremia present with increased ADH levels, beer potomania is unusual because the cause of hyponatremia is multifactorial, including low osmole intake. Furthermore, as these patients convert to a low ADH state, the rate of correction may be dramatic. As stated, our review of the literature found that 18% of patients presenting with beer potomania developed ODS. Patients who present with beer potomania are at greater risk of developing ODS because of the degree and chronicity of hyponatremia, alcohol use, and likelihood of correcting rapidly because of the underlying pathophysiological state. Because these patients often present with symptomatic severe hyponatremia, management can be challenging. Three large retrospective reviews of patients who presented with symptomatic severe hyponatremia (sodium ⬍ 120 mEq/L [mmol/L]) con- 678 Sanghvi et al cluded no benefit to aggressive correction of chronic hyponatremia.15,16,20 Furthermore, all 3 reviews showed a high complication rate of treatment (⬎5%) in patients presenting with chronic hyponatremia.15,16,20 The complication rate for patients with chronic severe hyponatremia correlated with both serum sodium level change greater than 0.55 mEq/L/h until serum sodium level was 120 mEq/L or greater (mmol/ L)20 and serum sodium level change greater than 10 mEq (mmol) in 24 hours15,16 and 18 mEq (mmol) in the first 48 hours.15 Animal studies showed a benefit in relowering serum sodium levels such that the net 24-hour change was less than 20 mEq/L (mmol/L),21 as well as benefit from relowering serum sodium level if there were neurological signs or symptoms consistent with ODS.22 Two documented clinical cases showed neurological benefit in relowering serum sodium levels after patients showed symptoms of ODS.23,24 Based on the underlying pathophysiological state and the literature reviewed, a table of recommendations (Table 2) and a treatment algorithm (Fig 2) are provided. It is well known that the most important risk factor for ODS is the rate of serum sodium level increase. Of the 22 cases of beer potomania reviewed, in 3 of the 4 cases resulting in ODS, serum sodium level increase in 48 hours was 20 to 24 mEq/L (mmol/L). Additionally, as stated, there were reports in animals and humans of benefit to relowering serum sodium levels.21-23 We believe that treatment goals for patients presenting with beer potomania should be a serum sodium level increase less than 10 Table 2. Management Recommendations Recommendations ● NPO except medications for 24 h ● No IVFs unless symptomatic ● Prescribe IVFs in finite amounts if needed ● Intensive care status ● Serum sodium every 2 h ● Concrete goals S Na increase ⬍ 10 mEq/L in first 24 h S Na increase ⬍ 18 mEq/L in first 48 h ● Relower serum sodium levels if necessary ● Give all IV medications in D5W ● If caloric intake is needed, use D5W Note: Sodium in mEq/L and mmol/L is equivalent. Abbreviations: NPO, nothing by mouth; IVF, intravenous fluids; IV, intravenous; D5W, dextrose 5% in water. mEq/L (mmol/L) in the first 24 hours and less than 18 mEq/L (mmol/L) in the first 48 hours (Fig 2). If the patient has neurological symptoms, correction of sodium level by 1 to 2 mEq/h (mmol/h) in the first 2 to 3 hours is reasonable. However, despite this initial more rapid correction, both the 24- (10 mEq [mmol]) and 48-hour (18 mEq [mmol]) goals should not be exceeded. If the patient is asymptomatic, fluid restriction and monitoring the patient despite the degree of hyponatremia is the recommended approach. If the serum sodium level increase occurs at a rate that will exceed the desired goal, D5W infusion should be started to match urine output. The D5W rate can be adjusted every 2 hours thereafter based on serum sodium level change. If serum sodium levels increase to greater than either the 24- or 48-hour goals, D5W rate should be increased to decrease the serum sodium level to the recommended goal. Desmopressin may be considered if diuresis occurs at an excessive rate that the infused D5W is unable to match; based upon the current rate of serum sodium level change, the goal will be exceeded despite D5W; the goal has been already been exceeded; or last, symptoms of ODS develop. Desmopressin was used successfully in 2 documented clinical cases.23,24 In both cases, patients presented with severe symptomatic hyponatremia. In both cases, patients had improvement in neurological symptoms, but later showed deterioration. In the first patient, symptoms included confusion and hyperreflexia, and in the second patient, quadriparesis and hyperreflexia were present. In the first patient, serum sodium level change was 21 mEq/L (mmol/L) in 24 hours, and in the second patient, serum sodium level changes at 24 and 48 hours were 12 mEq/L (mmol/L) and 23 mEq/L (mmol/L), respectively. Because of concerns of the role of rapid correction of serum sodium levels in the neurological deterioration, desmopressin and D5W were used to relower serum sodium levels. In both patients, mental status later improved. Although desmopressin poses its own risks, in the appropriate clinical setting with close monitoring, its use may benefit the patient. Our recommendations for the management of patients with beer potomania are listed in Table 2. Although the inclination is to feed these patients because of their malnourished state, our Risks and Management of Beer Potomania 679 Figure 2. Treatment algorithm. Note: Sodium in mEq/L and mmol/L is equivalent. Abbreviations: NS, normal saline; D5W, dextrose 5% in water; DDAVP, desmopressin; S Na, serum sodium. recommendation is to wait 24 hours because of the risk of osmole introduction and rapid correction. If caloric intake is needed, we recommend D5W. With fluid and feeding restriction, the patient’s serum sodium level likely will increase because of obligate solute losses and urinary electrolyte-free water loss. In addition, extrarenal free-water loss will contribute to the increase in serum sodium levels. If the patient’s sodium level does not increase, 0.45% NS solution can be prescribed, but a finite amount is recommended to prevent exceeding serum sodium goals. Conclusion Hyponatremia is a common biochemical abnormality presenting a therapeutic challenge to physicians. Beer potomania is a diagnosis of important consideration on initial evaluation of a patient presenting with hyponatremia. This syndrome of hyponatremia is unusual because patients have low levels of solute intake, and, if ADH secretion is not suppressed on admission, they likely will quickly convert to a state of low ADH secretion. The renal response to exogenous solute loads can be both dramatic and damaging. ODS is a known complication of overzealous treatment of patients with hyponatremia, but can occur even when the serum sodium level increase is in the historically acceptable range of less then 12 mEq (mmol) per 24 hours, especially in patients identified as high risk. Because ODS may not be recognized until 2 to 3 days after 680 Sanghvi et al the insult, treatment goals need to be identified at the initial evaluation. A table and algorithm are presented to detail the management and goals of treatment. If the syndrome of beer potomania is recognized and treatment goals are met, ideally, ODS can be avoided. ACKNOWLEDGEMENTS Support: None. Financial Disclosure: None. REFERENCES 1. Gwinup G, Chelvam R, Jabola R, Meister L: Beer drinker’s hyponatremia. Inappropriate concentration of the urine during ingestion of beer. Calif Med 116:78-81, 1972 2. Hilden T, Svendsen TL: Electrolyte disturbances in beer drinkers. A specific “hypo-osmolality syndrome”. Lancet 2:245-246, 1975 3. Swenson R, Rater DA: Electrolyte disturbances in beer drinkers. Lancet 1:372-373, 1976 (letter) 4. Liamis GL, Milionis HJ, Rizos EC, Siamopoulos KC, Elisaf MS: Mechanisms of hyponatraemia in alcohol patients. Alcohol Alcohol 35:612-616, 2000 5. Joyce SM, Potter R: Beer potomania: An unusual cause of symptomatic hyponatremia. Ann Emerg Med 15: 745-747, 1986 6. Demanet JC, Bonnyns M, Bleiberg H, StevensRocmans C: Coma due to water intoxication in beer drinkers. Lancet 2:1115-1117, 1971 7. Fenves AZ, Thomas S, Knochel JP: Beer potomania: Two cases and review of the literature. Clin Nephrol 45:6164, 1996 8. Kelly J, Wassif W, Mitchard J, Gardner WN: Severe hyponatraemia secondary to beer potomania complicated by central pontine myelinolysis. Int J Clin Pract 52:585-587, 1998 9. Leens C, Mukendi R, Foret F, Hacourt A, Devuyst O, Colin IM: Central and extrapontine myelinolysis in a patient in spite of a careful correction of hyponatremia. Clin Nephrol 55:248-253, 2001 10. Evans JR, Main J, Mitchell PE, Tulloch JA: Lactic acidosis and beer drinking. Scott Med J 30:237-238, 1985 11. Tien R, Arieff AI, Kucharczyk W, Wasik A, Kucharczyk J: Hyponatremic encephalopathy: Is central pontine myelinolysis a component? Am J Med 92:513-22, 1992 12. Decaux G, Soupart A: Treatment of symptomatic hyponatremia. Am J Med Sci 326:25-30, 2003 13. Arieff AI, Guisado R: Effects on the central nervous system of hypernatremic and hyponatremic states. Kidney Int 10:104-116, 1976 14. Dervisoglu E, Yegenaga I, Anik Y, Sengul E, Turgut T: Diffusion magnetic resonance imaging may provide prognostic information in osmotic demyelination syndrome: Report of a case. Acta Radiol 47:208-212, 2006 15. Sterns RH, Cappuccio JD, Silver SM, Cohen EP: Neurologic sequelae after treatment of severe hyponatremia: A multicenter perspective. J Am Soc Nephrol 4:1522-1530, 1994 16. Ellis SJ: Severe hyponatraemia: Complications and treatment. QJM 88:905-909, 1995 17. Thaler SM, Teitelbaum I, Berl T: “Beer potomania” in non-beer drinkers: Effect of low dietary solute intake. Am J Kidney Dis 31:1028-1031, 1998 18. Adrogue HJ, Madias NE: Hyponatremia. N Engl J Med 342:1581-1589, 2000 19. Laureno R, Karp BI: Myelinolysis after correction of hyponatremia. Ann Intern Med 126:57-62, 1997 20. Sterns RH: Severe symptomatic hyponatremia: Treatment and outcome. A study of 64 cases. Ann Intern Med 107:656-664, 1987 21. Soupart A, Penninckx R, Crenier L, Stenuit A, Perier O, Decaux G: Prevention of brain demyelination in rats after excessive correction of chronic hyponatremia by serum sodium lowering. Kidney Int 45:193-200, 1994 22. Soupart A, Penninckx R, Stenuit A, Perier O, Decaux G: Reinduction of hyponatremia improves survival in rats with myelinolysis-related neurologic symptoms. J Neuropathol Exp Neurol 55:594-601, 1996 23. Soupart A, Ngassa M, Decaux G: Therapeutic relowering of the serum sodium in a patient after excessive correction of hyponatremia. Clin Nephrol 51:383-386, 1999 24. Oya S, Tsutsumi K, Ueki K, Kirino T: Reinduction of hyponatremia to treat central pontine myelinolysis. Neurology 57:1931-1932, 2001








![NEC-255 PYRUVIC ACID, SODIUM SALT, [1- C]](http://s1.studyres.com/store/data/016736441_1-fc3f1c8fad455fdc5c1e9e44060828a8-150x150.png)