* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Infectious Sacro-Iliitis (ISI)
Survey
Document related concepts
Transcript
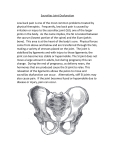
WScJ 1: 27-35, 2015 Infectious Sacro-Iliitis (ISI) Ahmad Morsi, Ahmed Sallam, Abdelfattah Saoud Department of Orthopaedic and Spine Surgery, Ain Shams University School of Medicine, Cairo, Egypt Abstract Infectious sacroiliitis is an uncommon disease that can affect different age groups. It is caused by multiple pathogens with variable clinical presentations and often non-specific laboratory results. A high index of suspicion should be maintained in patients presenting with low back or buttock pain to avoid misdiagnosis and delay in diagnosis. Early diagnosis with effective antibiotics, for the appropriate duration and through the appropriate route, will usually result in complete resolution with minimal chance of recurrence and complications. Surgical intervention in the form of drainage, debridement or arthrodesis can be needed for patients refractory to antibiotics, those with delayed diagnosis or those with generally compromised health states. Key words: Infection, sacro-iliitis Background I nfectious sacroiliitis (ISI) is an uncommon disease of variable presentation with various causative organisms. Pyogenic ISI (non-tuberculous, non-Brucella) accounts for 1.5% of pyogenic septic arthritis in children and less than 1% in adults (3,4). This is probably due to the poor vascularisation of this joint resulting in a low risk of infection via the haematogenous route (4). Diagnosis is usually difficult and delayed due to clinical heterogeneity and the lack of symptom specificity. Lack of awareness by clinicians, variable & poorly localizing signs of infection, frequent lack of systemic symptoms and serological criteria of infection makes it a “diagnostic challenge” (1-4,14). Most observations about infectious SI are based on case reports or small case series (4). Hermet et al (4), in 2012, presented the largest documented series of pyogenic ISI to date. They also reviewed cases published since 2009 (4). Three older studies examined isolated cases in reviews (4). The first review reported cases collected between 1878 and 1990, the second between 1990 and 1996, and the third between 1996 and 2009 (4). The most comprehensive review was that published World Spinal Column Journal, Volume 6 / No: 1 / January 2015 by Bernard Zimmermann III et al (14) in 1991 in which 166 cases were reviewed (4). Anatomy of the sacroiliac joint: Articular surfaces, nerve & blood supply. The sacroiliac joints (SIJ) are large central synovial articulations with a wide range of anatomic variation in the shape, extent of articular surface, and symmetry. Mobility of the SIJ is subject to some controversy, but most authors believe that there is some component of rotation around a transverse axis through the second sacral segment. Motion of the SIJ is greatest at birth and decreases from birth to puberty. In women, mobility increases after puberty to peak around 25 years of age. During pregnancy, the action of relaxin on ligaments and periarticular structures results in appreciable increase in sacroiliiac and pubic symphysis mobility. In men and women, mobility decreases in the fourth and fifth decades and is usually absent in the elderly (14). Innervation of the joint arises primarily from the lumbosacral plexus, which lies directly anterior to the joint capsule. SIJ vascularity originates from the pelvic and paravertebral venous plexus of Batson. The extent of vascularization of the joint has been shown to peak in the 27 Infectious Sacro-Iliitis (ISI) second decade of life and to decline after the age of 30. The composition of SIJ cartilage is similar to that of peripheral joints, although unique features of its collagen organization have been described (14). The variable clinical pictures associated with ISI may result from the characteristic anatomy of the SIJ. The first two sacral roots pass in close proximity to the SIJ, with the joint capsule adjacent to the psoas major muscle then the peritoneum anteriorly, and the gluteal and pyriformis muscles posteriorly. These relations might explain the anterior inguinal or abdominal pain, and the posterior buttocks or thigh pain depending on which part of the joint capsule is mainly involved (3,4). Pathogenesis: Causative pathogens and Risk factors Route of infection: Infectious agents may affect the joint in the form of reactive (immune-mediated) arthritis, or by hematogenous spread or direct penetration into the joint (14). Most SIJ infections appear to occur via a hematogenous spread (bacteraemia), although the frequency of obvious preceding infection varies (3,4,14). Previous reports have suggested that SIJ infections have a predilection for the iliac side of the joint, given the thicker cartilage barrier on the sacral side. Very rarely however, the periarticular abscess was confined to the sacrum (2). Age and gender: Multiple studies agree on the young age of prevalence of pyogenic ISI. While pyogenic ISI occurs more commonly in children and young adults, TB and brucellar ISI commonly affects young and middle-aged adults and their incidence in children and elderly people is low (12,14). The mean age of the 166 cases of pyogenic SI reported by Vyskocil et al. (12) and Zimmermann et al (14) were 22 and 20 years, respectively. Of the 343 patients in these two series, there were only 13 (4%) who were older than 60 years of age. It is possible that age-associated reduction in joint mobility or vascularization may decrease the probability of bacterial seeding of the joint space (3,4,14). Brucellar ISI was more frequent in the age group of 25-34 years (11,12). Gender predilection is variable in different case series. Pyogenic ISI may be slightly higher in females (3,4), while Tuberculous (TB) and Brucellar ISI may be more common in males (8,9). 28 During pregnancy and labour, the greater load demands on the SIJ (increased weight and hormone-induced changes) can explain the higher frequency of pyogenic ISI cases. These can occur during pregnancy, in the immediate postpartum period, or following abortion. Tuberculous ISI has also been reported in pregnant women (9). Causative organism: In pyogenic ISI, the definitive microbiological diagnosis may be based on blood cultures, joint fluid by CT-guided percutaneous puncture, or surgical specimens (4,14). When performed, blood cultures are positive in 42% to 69% (3,4) of adults and 45.5% of children (4). No primary source of infection can be identified in up to 40% of cases (14). More than 80% of reported cases of pyogenic ISI are caused by Gram-positive microorganisms, of which Staphylococcus aureus was by far the most common (4,14). Since 2007, Methicillin-resistant Staphylococcus aureus (MRSA) has been isolated as a cause of ISI (4). Streptococcal species were the second most common organisms, accounting for 9% of all cases. Streptococcus is the more frequent agent when gynaecological signs are present (4). Gram-negative infections were reported in 17% of all cases (14). Pseudomonas aeruginosa was the commonest Gram-negative bacillus in immunosuppressed and hospitalized patients, and predominantly intravenous drug users (4). Salmonella spp. was more frequent in children (4). Fewer cases of Escherichia coli ISI were reported, usually in conjunction with urinary tract infections (4,14). Till 2001, there have been only 17 cases caused by Salmonella reported in the English language (2). In contrast to streptococcal infection which was commonly associated with gynaecological signs of infection, diarrhoea or digestive problems were not systematically found when Salmonella was the causative agent (4). SIJ involvement has been reported in almost 10-11% of patients with skeletal tuberculosis and patients with systemic brucellosis. Fungal sacroiliitis caused by Cryptococcus neoformans has been reported in an immuno-suppressed patient (14). Side Predilection SIJ infections are usually unilateral and affect the left side in 60% of cases. An exception to that was a finding in a Taiwanese study, reporting a predominant right side affection or bilateral affection (4). Bilateral pyogenic World Spinal Column Journal, Volume 6 / No: 1 / January 2015 A Morsi et al. infections were reported during pregnancy (13.3% of cases) (4). Bilateral TB infections were rarely reported in patients with prolonged recumbency in supine position (8,9). Risk factors: Overall, risk factors are identifiable in 31% to 56% of patients (4). Vyskocil et al (12) reviewed 166 reported cases of pyogenic ISI and demonstrated that no associated factors or source of infection were noted in 41% of patients. This finding may even favor a delay in diagnosis (3). In adults, the most common predisposing factors are IV drug abuse and infections of the skin, respiratory and genitourinary tracts. In IV drug users, the SIJ is more commonly affected by pyogenic arthritis than any other joint, accounting for 24% to 39% of cases of joint infections in European heroin addicts (14). The following risk factors are linked to ISI (1-4,8,14): • Immune suppression (steroids, cytotoxic drugs). • Immune deficiency (uncontrolled diabetes, HIV, malignancy, hypogammaglobulinaemia). • Infective endocarditis, IV drug abuse. • Dental, cutaneous, respiratory or genitourinary infections. • Post-partum period (esp. after epidural anaesthesia for labour) • History of pelvic trauma (pyogenic / tuberculosis). • Sacroiliac joint injections, or faulty nearby IM injections. • Low socio-economic status, undeveloped country citizens (TB). • Farming, veterinarians, (Brucella). Laboratory workers Diagnosis: Diagnosis of ISI represent a “diagnostic challenge” and the delay in diagnosis of ISI can be explained by the atypical, non-specific and variable clinical symptoms and signs. The time to confirmed diagnosis is usually delayed reaching up to several months in some patients (1-4,14). Pyogenic ISI is diagnosed if there was bacteriological proof of infection or, in the absence of pathogenic agents, if the clinical, biological, and radiological data was compatible World Spinal Column Journal, Volume 6 / No: 1 / January 2015 with this diagnosis and evolution was favourable under antibiotic therapy (4). Unilateral sacroiliitis should always be considered infectious until proven otherwise (8). In some patients with pyogenic ISI, diagnosis is confirmed by prompt resolution of local symptoms and signs as well as systemic illness following antibiotic therapy (3). In contrast to pyogenic sacroiliitis, in which a high degree of suspicion can be obtained from positive blood culture results, diagnosis is much more difficult in sacroiliac tuberculosis (8). Diagnosis of brucella ISI can be suspected in areas in which brucella infection is endemic, and can be confirmed by a raised brucella titre. Clinical picture: Two major modes of presentation of pyogenic ISI have been defined. In the patients described by Vyskocil et al (12), 75% of the cases presented with acute onset of fever and severe continuous lower back pain exacerbated by motion or weight bearing and the patient may be unable to find a comfortable position (12). Fever can be present in 35-40% of cases (2 reports of fever in 75% of cases and 67% of pregnant women). Other patients experience the more gradual onset of symptoms, less pain, limping, painful hip extension and low-grade or absent fever. The vast majority of the reported patients with SIJ sepsis had unilateral infection, but symptoms may be diffuse and bilateral. Pelvic compression is usually painful. The Gaenslen and FABER maneuvers will often localize the SIJ as the source of pain. In a review of 191 cases of pyogenic ISI, 24 cases (13%) presented with acute abdominal pain which led to laparatomy in 10 cases. Pyogenic ISI in infants and young children may be difficult to distinguish from septic arthritis of the hip. Anterior expansion of the SI joint capsule may cause pressure on the iliopsoas muscle or sacral nerve roots, leading to radiating leg pain and weakness suggestive of lumbar disk disease (1-3,14). The first two sacral nerves cross anterior to the sacroiliac joint, and their irritation can cause radicular lower limb pain and can limit straight leg raising. The joint capsule is also bordered by the iliopsoas and piriformis muscles anteriorly and the gluteal muscles posteriorly. Therefore, if the joint capsule ruptures anteriorly, the irritation of the psoas muscles might cause hip pain, limping, and flexion 29 Infectious Sacro-Iliitis (ISI) deformity mimicking hip sepsis. In approximately 10% of all cases, disruption of the anterior capsule of the sacroiliac joint may result in peritoneal irritation and presentation of an acutely inflamed abdomen (3). Brucella ISI: Turan et al (11) reported that osteoarticular involvement (including SIJ) was more common in subacute and chronic cases than in acute cases (presenting within 8 weeks of symptom onset). Fever, chills, shivering, excessive sweating, muscle, and joint pain are the most common symptoms in acute and subacute cases (3,4). Turen et al (11) reported that the SIJ is the most commonly affected joint by brucellosis (60.6%), and that 82.4% of patients with brucellar ISI were bilateral. Commonly, history included employment in farming and/or consumption of un-pasteurized milk and dairy products, especially fresh cheese. Hepatosplenomegaly was more markedly associated when osteoarticular affection was evident (11). Presentation in pediatric versus adult populations: Children: ISI is rare in children and even more challenging at diagnosis. Younger uncooperative children show highest incidence in prolonged delay in diagnosis (13). In 2007, Wu et al (13) retrospectively reviewed 33 patients (11 children; <15 yrs of age and 22 adults; >=16 yrs of age) who had been diagnosed between 1996 and 2005 with pyogenic SI. This was a relatively large case series that compared presentations in pediatric and adult populations. Among the all included patients, females were attacked more frequently than males (3: 1). One-third of patients had concurrent infections, of which urinary tract infections were the most common (41.6%). Secondary inability to walk and/ or pseudo paralysis should raise index of suspicion towards SIJ affection in children (13). Compared with adult patients, pediatric patients tend to have fewer comorbid immune-compromised conditions, fewer concurrent infections, more equality in gender distribution and more presentations of weight bearing difficulty. Staphylococcus aureus was the main blood culture isolate from pediatric patients (80%), but only accounted for 50% of those from adult patients. Group B streptococcus and Salmonella spp. were not uncommon in the adult patients (13). Differential diagnosis: On admission, only few cases are suspected to have ISI. Other suspected diagnoses on admission include: lumbar 30 disc herniation with or without sciatica (commonest), inflammatory and reactive sacroiliitis (Table 1), crystal deposition arthritis (gout/pseudogout), degenerative disc disease, spondylodiscitis, mechanical low back pain, septic hip arthritis, psoas abscess or hematoma, sigmoiditis, sacral insufficiency fractures or sacral metastasis (4,14). Traumatic injuries of the SI joint are seen most commonly after violent injuries. Metastatic carcinoma (9) or sarcoma (12) may rarely mimic inflammatory sacroiliitis. Degenerative changes of the SI joint have been associated with aging (14). Table 1: Inflammatory SIJ arthritis Inflammatory SIJ arthritis (14): • • • • • • • Sero-negative spondyloarthritis Rheumatoid arthritis Familial Mediterranean fever Hyperparathyroidism Relapsing polychondritis Behcet’s disease Whipple’s disease Bedside tests: Tuberculin test: Tuberculin skin testing can be helpful in diagnosis, however, a positive response is not specific for current infection, and rarely, the test result may be negative in active disease. A previous history of incompletely treated tuberculosis, no matter how remote, should be highly suggestive of infection, and should always be sought in this context (8). Serological testing: Most serological tests are sensitive but non-specific in diagnosis of ISI (14). These inflammatory markers can help in diagnosis and in follow-up of treatment. ESR and CRP are always elevated in all types of ISI (pyogenic, brucellosis and TB) (1). Fibrinogen levels are often elevated (>25% of patients) and leucocytic count can show leucocytosis (polymorphs) in 40% of patients with pyogenic ISI (4). Still, the leucocytic count can be within normal range in many patients (1,2). Tuberculous and Brucellar ISI patients may show mild anaemia of chronic disease and some may show leucopenia. Brucellar ISI can be frequently associated with lymphocytosis, thrombocytopenia, pancytopenia or DIC (8,12). World Spinal Column Journal, Volume 6 / No: 1 / January 2015 A Morsi et al. Brucellar ISI when suspected, can be confirmed by standard tube agglutination (STA) test. A titre > 1/160 or a four-fold increase between 2 occasions 2-3 weeks apart are diagnostic of brucellar infection. Moreover, hepatic enzymes were found to be moderately elevated in cases of brucellar ISI (21%) (12). Bacteriological examination: Examination of joint aspirate, abscess aspirate, blood samples in the form of stained films and culture and antibiotic sensitivity tests will confirm diagnosis, specify the organism and indicate appropriate antibiotic regimen. Gram stained films will demonstrate the causative organism in pyogenic ISI (cocci/bacilli) and Brucella (Gram negative coccobacilli). TB acid fast bacilli can be demonstrated using the Ziehl-Neelsen stain (8). Sputum and urine specimens may help guide the diagnosis when stained films and cultures are positive. Mycobacterium tuberculosis can be grown on specific media in 4 to 6 weeks but treatment should be started once clinical suspicion of TB infection is high. If blood culture is positive for bacteraemia, (around 40% of pyogenic and less in brucella cases), joint aspiration (arthrocentesis) may not be necessary. Otherwise, CT or fluoroscopy guided aspirate should be obtained and stained films and proper culture media should be used to avoid missing atypical organisms (4,14). The frequency of pyogenic ISI without any identified pathogenic agent has tended to decrease over time, from 27% in the literature review of Mancarella et al in 2009 (6) to 15.4% in the study of Hermet et al in 2012 (4). In such cases, and in all cases while waiting for culture results, parenteral combined regimens of broad spectrum antibiotics should be used. The appropriate antibiotic therapy should be started after confirmation of the causative organism. Turin et al (11) found that the mean time of growth of Brucella melitensis (commonest species) in blood cultures in patients with osteoarticular involvement (60.6% of which were sacroiliitis) was 5.2 days, a time significantly shorter compared to patients without osteoarticular involvement. A definite diagnosis of brucellosis can be made by isolating Brucella spp. from blood, bone marrow or other tissues. However, isolation of the microorganism in blood culture is difficult and obtaining bone marrow and tissue culture is invasive and clinically impractical. Thus confirmation of diagnosis is usually based on serum antibody detection (11). World Spinal Column Journal, Volume 6 / No: 1 / January 2015 Histo-pathological examination of specimens obtained from tuberculous ISI patients can demonstrate granulomatous changes or caseous necrosis compatible with tuberculosis. Some samples may show non-specific inflammatory changes. Imaging: The availability of newer diagnostic imaging techniques has greatly improved the ability to diagnose and assess SIJ infections (1). Plain Radiographs: In the early and acute stage of pyogenic infections, plain radiographs are usually normal (1,4), while in chronic cases, they can show widening of the joint space, periarticular osteopaenia, sclerosis and blurring of subchondral plate, and erosion of the joint margins (1). However, these abnormalities are not likely to appear until several weeks after the onset of pyogenic infection (14). In contrast, these findings may show on plain radiographs performed on admission of TB SI (8,9) and in subacute and chronic cases of brucella SI (3). The radiographic abnormalities in sacroiliac tuberculosis are identical to those observed in late pyogenic sacroiliitis. In TB SI, plain radiographs obtained at admission are usually abnormal. The majority will show joint space widening and bony erosions of both sacral and iliac joint margins. Both juxta-articular sclerosis or periarticular demineralization have been reported (8). There is no evidence from the literature that scintigraphy, computed tomography, or magnetic resonance imaging differentiate pyogenic from granulomatous sacroiliitis (8). Computed Tomography: Occasionally, CT scanning has shown changes suggestive of septic sacroiliitis at a time when technetium scans and plain radiographs were normal. Still, many CT scans remain normal especially in early pyogenic ISI (3). In advanced cases, pelvic and abdominal CT scans can show bony changes (SI joint destruction) as well as soft tissue and intramuscular abscesses (psoas, iliacus, gluteal, intrapelvic) (3,4). CT-guided aspiration (arthrocentesis) is beneficial in specifying the causative organism in most cases and is indicated when blood cultures fail to show the type of organism (negative blood cultures in almost half of pyogenic cases, and in the majority of TB and brucella cases (11). 31 Infectious Sacro-Iliitis (ISI) In chronic cases (which usually need surgery), preoperative CT can show joint space widening, sclerosis of the margins of the joint, cavitations, sequestrum formation and extent of bone destruction. Thus it can help decision making as regards approach for debridement and need for arthrodesis (1). Post-operatively, CT is useful in assessment of bony fusion, usually when patients remain symptomatic (1). Scintigraphy: Technetium (Tc-99) bone scanning is more sensitive for the diagnosis of SI joint infection than plain radiography and may be positive as early as 3 days after the onset of symptoms (3). However, bone scanning may return negative results if performed too early after the onset of symptoms (3). Although bone scans are not specific, they may be useful in localising the infection when no clinical localization is possible (4). Gallium-67 citrate scanning was shown to demonstrate uptake positivity earlier than Tc-99 scanning in a group of drug users with septic arthritis (14). Contrary to most other studies, Wu et al denoted that scintigraphic bone scan has the highest sensitivity (93.3%) and remains the image modality of choice and that when local abscess formation is suspected, computed tomography or magnetic resonance imaging may be the preferred method used for examination. Skeletal scintigraphy Tc99 or Ga67 can be very helpful in diagnosis and infection localization when febrile illness cannot be attributed to a specific body system in a young child (13). Bone scans have 3 main disadvantages: the inability to differentiate infectious from non-infectious sacroiliitis, the inability to differentiate sacroiliitis from psoas or gluteal abscess and the inability to identify spread of the infection from the joint into the surrounding tissues (1,3). Magnetic resonance imaging (MRI): MRI is the reference examination for establishing the diagnosis of ISI. It also enables clinicians to assess whether the infection has spread to the adjacent muscular structures (4). MRI can provide more diagnostic accuracy in pyogenic sacroiliitis than CT or radionuclide scanning (3). It can show bone marrow edema, fluid and pus collections inside and adjacent to the joint (gluteal, iliacus, psoas, pyriformis, intrapelvic). Gadolinium (Gd-DTPA) can show enhancement in the SI joint space, adjacent bone marrow (usually more extensive on the iliac side), and muscles (1,3). 32 In the chronic phase, MRI can show periarticular bone marrow reconversion, replacement of articular cartilage by pannus, bone erosion, subchondral sclerosis, joint space widening or narrowing and ankylosis (1). Hermet et al (4) recommended that MRI of the lumbar spine and SI should be systematically performed in a febrile patient with lumbogluteal pain, particularly in the case of pregnancy. MRI signal anomalies persist for several months, even when clinical and biological improvement appears promising (4). Klein et al stated that in six septic SI joints studied, all had positive MRIs, five had positive gallium scans, three had positive CT scans and one had a positive technetium scan (5). Murphey et al showed that MRI was superior to CT for evaluation of cartilage integrity and osseous erosions in patients with inflammatory and infectious sacroiliitis. MRI saves the patient exposure to ionizing radiation and is thus beneficial in evaluation of pregnant women (7). Although there are no typical MRI features differentiating infectious from non-infectious sacroiliitis (4), findings favouring the diagnosis of infectious sacroiliitis were: unilateral disease and prominent soft tissue and marrow edema adjacent to the SI joint (14). Recently, Positron emission tomography (PET/CT) with fluorine-18 fluorodeoxyglucose appeared to be an interesting technique for the very early diagnosis of ISI, even before MRI reveals any abnormalities (4). Treatment: ISI presents a diagnostic rather than a therapeutic problem, because the timely administration of antibiotics usually leads to resolution without surgical intervention in the majority of cases (3). Antibiotic therapy choice: If the infection is diagnosed at an early stage, empirical combination of broad-spectrum antibiotics should be parenterally administered until blood or joint aspirate culture results are available (3). Once the diagnosis is confirmed and the causative organism is specified, culture specific parenteral antibiotic therapy should be started until symptoms and signs significantly subside. Afterwards, patients can be discharged home on the appropriate oral antibiotic regimen which can World Spinal Column Journal, Volume 6 / No: 1 / January 2015 A Morsi et al. continue for weeks to months and are not stopped until the inflammatory markers have returned to normal. If culture results are negative, empirical anti-Staph aureus antibiotics (in non-addicted population), should be administered (3,4). In the case of failure to improve symptoms and signs within 48 hours, anti- Gram-negative bacilli antibiotics should be added, in line with the SPILF (Société de Pathologie Infectieuse de Langue Française) recommendations for spondylodiscitis (4). Table 2 shows suggestions for specific effective antibiotic combinations: For Brucellar ISI, Turan et al (11) recommended double or triple antibiotic combinations depending on the clinical signs and complications. This included double antibiotic combination of rifampicin 600 mg/day and doxycycline 200 mg/day; with streptomycin one g/day and doxycycline 200 mg/day or triple antibiotic combination with rifampicin 600 mg/day, doxycycline 200 mg/day and streptomycin one g/ day (21 days) (11). For Tuberculous ISI: Triple antibiotic combination (2 first line and 1 second line drugs) is prescribed for the first 2 months followed by at least 7 to 10 months of dual combination (2 first line drugs). First line drugs include: Rifampicin and Isoniazid. Second line drugs include Pyrazinamide, Ethambutol and Streptomycin Duration of antibiotic therapy: There are no clear guidelines in the literature indicating the optimal duration of intravenous and oral antibiotic therapy. Most authors suggest a minimum of 2-3 weeks intravenous and 3-6 weeks oral antibiotics in cases of pyogenic ISI (3,4,14). Hermet et al stated that “it seems reasonable to propose parenteral treatment for 2 weeks followed by oral treatment for 6 weeks in the case of pyogenic SI, which is in accordance with the SPILF recommendations for the treatment of infectious spondylodiscitis” (4). However, British recommendations for the treatment of infectious spondylodiscitis favour parenteral treatment for 3 weeks followed by oral treatment for a total of 6–12 weeks. Hermet et al (4) noted that antibiotic therapy for more than 6 weeks does not reduce the risk of relapse. Therefore, there appears to be no justification in prolonging treatment beyond 6 weeks (4). M.-S. Wu et al 2007 recommended IV antibiotics in children for at least 20 days to prevent relapses (13). For brucellar ISI, Turan et al recommended that treatment should continue for 3 months in cases of osteo-articular brucella involvement (of which ISI represented 60.9%) (11). For Tuberculous ISI, treatment should continue for 9 to 12 months. Few studies have continued therapy for only 6 months with favourable outcomes. Resolution of infection: Infection of the SIJ is considered to be healed by the disappearance of clinical symptoms (pain, local tenderness, fever, etc.) and normalization of laboratory parameters of infection (WBC, CRP and ESR) (1). If parenteral antibiotics fail to show appreciable improvement in the patient’s signs and symptoms within 48 hours, surgical drainage and debridement should be considered. This is true for pyogenic and brucellar SI. Response to antibiotic treatment in tuberculous SI is usually slow as the disease itself is usually of chronic course. Surgical treatment: Indications for surgical drainage include abscess formation, evidence of contiguous osteomyelitis, and sequestrum of necrotic bone, septicaemia, neurological deficits and failure to respond to antibiotic therapy within 48 hours (1,2,4,14). In such cases, surgical drainage will almost always lead to complete the resolution of infection. It is to be stated that before the antibiotic era, septic sacroiliitis Table 2: Antibiotic Suggestions for different pathogens. Pathogen Staph. aureus: MRSA (Coagulase negative): Antibiotic Combinations Rifampicin, oxacillin, ofloxacin Vancomycin Amoxicillin+/-Clavulanic acid, Gentamycin, Ceftriaxone, Ofloxacin, Streptococci: Cefotaxime Pseud. aeruginosa: (IV drug abusers till culture) Tazocilline, Ciprofloxacin, Colistin, Ceftazidime, Amikacin. E. coli: Amoxicillin + Rifampicin World Spinal Column Journal, Volume 6 / No: 1 / January 2015 33 Infectious Sacro-Iliitis (ISI) Figure 1: Treatment algorithm in infectious sacroiliitis. produced large abscesses and had a reported 30% to 40% mortality rate (2). Surgical intervention included debridement with or without joint arthrodesis. The surgical approach was either posterior, anterior or combined anterior and posterior. The localisation of the infection (abscess and soft tissue infiltration) as demonstrated by MRI dictated the operative approach (1). If there is evidence of abscess formation posterior to the joint, or osteomyelitis or necrotic sequestrum of bone within the joint, an incision over the posterior aspect of the SI joint will allow full access for drainage of the abscess and debridement of necrotic bone and debris. In cases in which the distended SI joint capsule protrudes anteriorly into the pelvis, the retroperitoneal and retrofascial spaces must be explored (14). Postoperative treatment included culture-based antimicrobial therapy or broad-spectrum antibiotic therapy when no organism was isolated, for 6 weeks in non-specific infections and 6–12 months in tuberculous infections (1). The postoperative immobilisation period depended on the general condition of the patient and the operative technique (1). 34 Ahmed et al studied 22 patients with ISI (tuberculous and pyogenic) who needed 24 surgeries and demonstrated that surgical outcome was favourable and that 2 patients required revision surgery to induce fusion after infection has recurred after complete resolution of infection (1). Their complications also included delayed wound healing in three cases and chronic pain in three cases (1). There is debate over whether to perform arthrodesis of the joint or to limit surgery to drainage of the abscess and debridement of the joint. Ahmed et al (1), recommended that the operative management of SIJ infections consists of debridement in cases of acute soft tissue infection or cases of mild bone destruction and that joint arthrodesis should be performed in generally ill patients even with mild joint destruction as well as in patients with chronic joint affection. This will help early assisted mobilization. Figure (3) shows an algorithm Suggested by Ahmed et al (1) for surgical decision making in cases of ISI. If SIJ stabilization is needed, as in cases where primary arthrodesis is indicated, or cases when chronic SIJ pain results from joint damage after infection, a minimally invasive procedure using with titanium bi-iliac screws and transverse World Spinal Column Journal, Volume 6 / No: 1 / January 2015 A Morsi et al. rods can be used. This technique (The novel pelvic Internal fixator) has been introduced by Saoud and Reda (10) in 2011 and was primarily indicated for stabilizing transforamenal sacral fracture REFERENCES 1. Ahmed H, Siam AE, Gouda-Mohamed GM, et al. Surgical treatment of sacroiliac joint infection. J Orthopaed Traumatol 2013; 14:121– 129. 2. Attarian DE. Septic Sacroiliitis: The Overlooked Diagnosis. J South Orthop Assoc. 2001: 10. 3. Doita M, Yoshiya S, Nabeshima Y, et al. Acute Pyogenic Sacroiliitis Without Predisposing Conditions. SPINE 2003; Volume 28, 384–389. 4. Hermet M, Minichiello E, Flipo RM, et al. Infectious sacroiliitis: a retrospective, multicentre study of 39 adults. Infectious Diseases 2012; 12:305. 9. Ramlakan RJ, Govender S. Sacroiliac joint tuberculosis. International orthopedics 2007, 31: 121-124. 10. Saoud AM, Abdelwahab MR. The Internal Fixator: A Novel Technique for Stabilization of Transforaminal Sacral Fractures as a Part of Pelvic Ring Disruption. A Preliminary Report. 2011: Vol 2 (1): 027-036 11. Turan H, Serefhanoglu K, Karadeli E, Togan T, Arslan H. Osteoarticular Involvement among 202 Brucellosis Cases Identified in Central Anatolia Region of Turkey. Intern Med 2011: 50: 421-428. 12. Vyskocil JJ, McIlroy MA, Brennan TA, et al. Pyogenic infection of the sacroiliac joint. Case reports znd review of the literature. Medicine 1991, 70(3): 188-197. 13. Wu MS, Chang SS, Lee SH, et al. Pyogenic sacroiliitis: a comparison between paediatric and adult patients. Rheumatology 2007; 46: 1684– 1687. 14. Zimmermann B 3rd, Mikolich DJ, Lally EV. Septic Sacroiliitis. Semin Arthritis Rheum 1996: 26: 592-604. 5. Klein MA, Winalski CS, Wax MR, et al: MR imaging of septic sacroiliitis. J Comput Assist Tomogr 1991: 15: 126-132. 6. Mancarella L, De Santis M, Magarelli N, et al. Septic sacroilitis: an uncommon septic arthritis. Clin Exp Rheumatol 2009; (6)1004:1008. 7. Murphey MD, Wetzel LH, Bramble JM, et al. Sacroiliitis: MR imaging findings. Radiology 1991: 180: 239-244. 8. Pouchot J, Vinceneux P, Barge J, et al. Tuberculosis of the Sacroiliac Joint: Clinical Features, Outcome, and Evaluation of Closed Needle Biopsy in 11 Consecutive Cases. The American Journal of Medicine 1988, 64: 622-628. World Spinal Column Journal, Volume 6 / No: 1 / January 2015 Manuscript submitted January 27, 2015. Accepted January 31, 2015. Address correspondence to: Abdelfattah Saoud, Department of Orthopaedic and Spine Surgery, Ain Shams University School of Medicine, cairo, Egypt email: [email protected] 35