* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Using Vectorcardiography In Cardiac Resynchronization Therapy
Survey
Document related concepts
Transcript
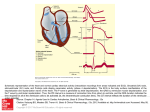
Using Vectorcardiography In Cardiac Resynchronization Therapy By L.Lindeboom BMTE 10.19 Report Internship Catharina Hospital Eindhoven Eindhoven University of Technology Supervisors Dr. Ir. M. van ‘t Veer Dr. B.M. van Gelder Dr. Ir. M.C.M. Rutten Prof. Dr. N.H.J. Pijls 1 Abstract (English) The conductive system of the heart may be affected by a heart disease due to direct damage of the Purkinje bundle branches or by a changed geometry in a dilated heart. As a result the electrical activation impulse will no longer travel across the preferred pathway and a loss of ventricular synchrony, prolonged ventricular depolarization and a corresponding drop in the cardiac output is observed. During cardiac resynchronization therapy (CRT) a biventricular pacemaker is implanted, which is used to resynchronise the contraction between different parts of the myocardium. Optimize pacing lead placement and CRT device programming, is important to maximize the benefit for the selected patients. The use of vectorcardiography (VCG) for CRT optimization is investigated. In current clinical practice a 12-lead electrocardiogram (ECG) is used to measure the electric cardiac activity of a patient. Each cell in the heart can be represented as an electrical dipole with differing direction during a heartbeat. A collection of all cellular dipoles will result in a single dipole, the cardiac electrical vector. Spatial visualization of the intrinsically threedimensional phenomena, using VCG, might allow for an improved interpretation of the electric cardiac activity as compared to the one dimensional projections of a scalar ECG. The VCG loops of one healthy subject and two subjects with a left bundle branch block (LBBB) and two subjects with a right bundle branch block (RBBB) are qualitatively described. It is shown that different electrical activation patterns will indeed result in different VCG’s and that the VCG loops give an intuitive insight into the conductive pathways. Differences in VCG loops after right ventricular and left ventricular pacing and the influence of lead placement are analyzed. (Dutch - Samenvatting) Door directe of indirecte schade aan het elektrische geleidingssysteem van het hart, bereikt de elektrische prikkel verschillende delen van het hart niet gelijktijdig, waardoor de samentrekking van het hart niet synchroon zal plaatsvinden. Door het implanteren van een biventriculaire pacemaker wordt getracht het hartspierweefsel op verschillende plaatsen, met verschillende tijdsintervallen, elektrisch te stimuleren om het samentrekken van het hart te resynchroniseren. Het gebruik van vectorcardiografie (VCG) bij het zoeken naar de optimale plaatsing en de optimale tijdsinstellingen van de pacemaker, wordt onderzocht. Elke cel in het hart kan worden gezien als een kleine elektrische dipool, verschillend van grootte en richting gedurende een hartcyclus. Door het optellen van alle dipolen ontstaat de elektrische hartvector. Met gebruik van het standaard 12-afleidingen electrocardiogram (ECG), is het mogelijk om de elektrische hartvector uit te rekenen en weer te geven in een vectorcardiogram, waarbij de twaalf figuren van het ECG worden gereduceerd tot één figuur voor het VCG. De VCG’s van één gezond testpersoon, van twee testpersonen met een linkerbundeltakblok en van twee testpersonen met een rechterbundeltakblok worden kwalitatief beschreven en vergeleken. De invloed van rechter- en linkerventrikel pacing wordt tevens beschreven. De VCG’s lijken een goed inzicht te geven in de geleiding van de elektrische prikkel over het hart. 2 Contents 1. Introduction 1.1 Background 1.2 Cardiac Resynchronization Therapy 1.3 Electrocardiography 1.4 Description of Vectorcardiogram 1.4.1 Healthy subject 1.4.2 LBBB 1.4.3 RBBB 1.4.4 Pacing 1.5 Project goal 2. Materials and Methods 2.1 Patient population and data acquisition 2.2 Signal analysis 2.2.1 Filtering of the ECG signal 2.2.2 Calculation of the VCG 2.2.3 Definition of an average heartbeat 2.2.4 Differentiation of the depolarization wave 2.3 Qualitative measures of the VCG 2.4 Quantitative measures of the VCG 2.4.1 Mean electrical axis 2.4.2 VCG loop area 2.4.3 Duration of depolarization 3. Results 3.1 Measurements 3.2 Healthy subject, LBBB subjects and RBBB subjects 3.2.1 Healthy subject 3.2.2 Bundle branch blocks 3.2.3 Overall observations 3.3 RV and LV pacing 3.3.1 RV pacing 3.3.2 LV pacing 3.3.3 Overall observations 4. Discussion and Conclusions 4.1 Healthy VCG 4.2 VCG of LBBB intrinsic and with pacing 4.3 VCG of RBBB intrinsic and with pacing 5 5 6 6 7 7 9 9 10 11 12 12 12 12 12 13 13 15 15 15 16 16 17 17 17 17 18 20 20 20 21 22 23 23 23 23 3 4.4 Pacing lead position 4.5 Normalization of the VCG 4.6 VCG in CRT 4.7 Quantification of the VCG 4.8 Repolarization 4.9 Summary of conclusions 23 24 24 25 25 25 5. Future Directions 27 6. References 28 Appendix A – ECG’s Appendix B – 3D Representations of VCG loops Appendix C – 2D Representations of VCG loops 29 36 43 4 1. Introduction 1.1 Background Heart failure, defined as the inability of the heart to supply sufficient blood flow to the organs in the body, is an important cause of hospitalization in patients older than 65 years. Due to the impairment of the cardiac output, patients suffer from breathlessness and fatigue. Initial causes for heart failure include hypertension, valvular heart disease, cardiomyopathy, and ischemic heart disease, often accompanied by dilatation of the ventricles of the heart and alterations in the electrical activation patterns [1]. The heart is endowed with a system for the generation and conduction of electrical impulses to cause rhythmical and synchronic contractions of the heart muscle (see figure 1). Initially the rhythmical electrical impulses are generated in the sinus node (or S-A node) and travel via the atria towards the atrioventricular node (A-V node). Special Purkinje fibers lead the impulses from the A-V node through the A-V bundle into the ventricles. These fibers divide into a left and a right bundle branch. Each branch spreads downward towards the apex of the heart, progressively dividing into smaller branches. The ends of the Purkinje fibers penetrate about one third of the muscle mass. Because the speed of transmission of the impulse in these Purkinje fibers is about 5 times higher than transmission through the heart muscle Figure 1 The cardiac rhytmical excitation system itself, the conduction system causes the (Adapted from Textbook of Medical Physiology Eleventh Edition, Guyton and Hall, Chapter 10) electrical impulse to arrive at almost all segments of the ventricles within a narrow time span resulting in a synchronous contraction. This is required for an effective pumping by the two ventricular chambers of the heart [2]. In a number of patients suffering from heart failure the conduction system might be affected due to direct damage of the conductive system by myocardial ischemia or by the changed geometry in the dilated heart. In these cases the bundle braches of the Purkinje system become diseased or damaged and will stop to conduct the electrical impulses. Since the electrical impulse can no longer travel across the preferred pathway, it will move through the muscle fibers, which slows the electrical conduction and which changes the directional propagation of the impulse. The impulse will arrive at different segments of the heart with an increased time interval. A differentiation between a left of a right bundle branch block (LBBB and RBBB respectively) is made. As a result of the BBB, there is a loss of ventricular synchrony, ventricular depolarization is prolonged and a corresponding drop in the cardiac output is observed. 5 1.2 Cardiac Resynchronization Therapy Cardiac resynchronization therapy (CRT) aims to reduce the symptoms of breathlessness and fatigue in patients suffering from heart failure, with delayed and dyssynchronous left ventricular contraction as a result, by restoration of a more physiological sequence in cardiac activation. Resynchronisation is achieved by the implantation of a biventricular pacemaker or biventricular ICD (implantable cardioverter defibrillator) which enables activation of the myocardium at different locations and with different time delays (see figure 2). The goal of CRT is to activate both ventricles simultaneously to restore the synchrony between the ventricles [1]. To predict the optimal lead placement Figure 2 Graphical representation of the heart in a section and the optimal time interval between perpendicular to the septum of the heart. The red dots indicate the the pacing electrodes currently regions for possible lead positions. echocardiography and measurements of pressure build-up are used. Echocardiography is used to identify mechanical dyssynchrony with the use of tissue Doppler imaging (TDI), which measures the systolic and diastolic velocity in different segments of the myocardium. A notable regional variation in velocities is a typical appearance in patients with dyssychronous ventricle contraction. This method requires experience in echocardiography and is very time consuming and rather operant dependent [3]. A more objective and reproducible method is to measure the amount of pressure increase per unit of time (or dP/dt) in the left ventricle, which evaluates the pumping effectiveness of the heart. An optimal lead placement and an optimal timing interval between the pacing electrodes for the biventricular pacemaker are found with a maximal value in dP/dt [4]. Several randomized controlled trials and numerous observational studies have demonstrated improvements in exercise capacity and quality of life after CRT procedures in patients suffering from heart failure. Despite these advances approximately 25% of patients who meet current criteria for implantation of a CRT device do not show objective evidence of clinical benefit (the ‘non-responders’) [3]. Implantation of a CRT device is expensive, time consuming and involves invasive medical surgery. It is important to optimize pacing lead placement and device programming to maximize the benefit for the selected patients. Alternative methods for optimization are investigated. 1.3 Electrocardiography In current clinical practice a standard 12-lead electrocardiogram (ECG) is used to measure the electric cardiac activity of a patient. The change in the electrical activation of the heart caused by a LBBB and a RBBB are recognized as different waves in the different 6 electrocardiographic leads. Besides these intrinsic conduction disorders, different activation patterns as a result of different pacing lead positions in CRT will also result in different ECG’s. In a standard 12-lead ECG a projection of the electric cardiac vector is drawn along standardized leads. The connections for the standard ECG are shown in figure 3. The electric cardiac vector is a superposition of the numerous electrical dipoles that exist during the depolarization of the cardiac muscle at each point in time. This is further explained in paragraph 1.4. The voltage which is recorded in a normal ECG depends on the placement of the electrodes on the body surface of a patient, the amount of excited cardiac muscle and the body composition of the patient. Because of resistance of the tissue between the heart and the skin, the measured signal will decrease with an increasing distance from the heart muscle. Figure 3 Arrangement of electrodes for a standard Because the separate leads in the ECG give a projection 12-lead ECG of the cardiac electrical vector along one single direction (Adapted from Textbook of Medical Physiology Eleventh Edition, Guyton and Hall, Chapter 11) spatial information is difficult to interpret from 12 different images. Thereby, redundant information is presented in a 12-lead ECG. Spatial visualization of the intrinsically three-dimensional phenomenon in a single image might allow for an improved interpretation of the electric cardiac activity as compared to the one-dimensional projections of a scalar ECG. In 1956 Frank developed a method with which he was able to obtain a 3D representation of the electrical activity of the heart, represented by a right-left axis (X), a head-to-feet (cranial to caudal) axis (Y) and a front-back (anterior to posterior) axis (Z) [5]. Frank used seven electrodes to calculate the X, Y and Z potentials. The electrode placement and the X, Y and Z directions are shown in figure 4. From the scalar X, Y and Z coordinates it is possible to obtain the instantaneous cardiac vector, whose path in space builds the vectorcardiographic loop or a vectorcardiogram. 1.4 Description of the Vectorcardiogram 1.4.1 Healthy subject Figure 5 shows the depolarization of the heart for a healthy subject, together with the expected ECG and VCG. The figure shows the result of the depolarization on the cardiac vector in the frontal plane as indicated by the red axes in figure 5A. Figure 4 Frank vectorcardiographic lead system. Seven electrodes used are denoted with A, C, E, I, M, H and F. 7 Figure 5 Schematic drawing of different time steps of the cardiac electrical impulse pathway during depolarization for a healthy subject in the frontal plane as indicated by the red arrows in panel A. The expected VCG and ECG (shown under the heart respectively) are plotted for successive time steps in panel B-F. While the impulse travels over the heart, the resultant vector changes in direction and magnitude. Tracking the direction and magnitude of the resultant vector in time results in the VCG loop. The ECG only gives the magnitude of the vector. The small loop indicated with “p” within the larger VCG loop represents the electrical activity of the atria. (Adapted from 'Presentation and Analysis of Vector Electrocardiograms', Anna Redz, March 1998) As the impulse travels from the A-V node into the ventricles through the Purkinje system, the impulse arrives in the left bundle branch slightly before the right bundle branch. Depolarization starts from the left side of the septum. Initially the cardiac vector is pointing from left to right, which is visible in a vector pointing to the right in the VCG (figure 5B). Then the vector changes direction as the depolarization wave expands towards the apex and the LV and RV (figure 5C). Because the left ventricle consists of more muscle mass, it will take slightly longer to completely depolarize this ventricle. The vector in the VCG is a resultant vector from multiple existing dipoles over the heart, with the larger potential differences appearing in the left ventricle. Hence, the resultant vector in the VCG will therefore mainly be directed to the left (figure 5D and 5E). Furthermore the loop will mainly be directed caudal as can be seen by the vector pointing towards the apex (figure 5C and 5D). When the heart is completely depolarized, no dipoles will exist and the loop is closed. In the horizontal plane (defined by the x- and z-axis, see figure 4) the left ventricle is positioned behind the right ventricle. Because larger potential differences appear in the left ventricle the main direction of the loop in 3D will be posterior. 8 1.4.2 LBBB Figure 6 Schematic overview of depolarization with LBBB in the frontal plane, as indicated by the red arrows in A. The right ventricle will depolarize before the left ventricle. The impulse will travel via the apex of the heart towards the left ventricle resulting in a mainly cranial oriented VCG (C and D) Figure 6 gives a schematic overview of the depolarization for a subject with a LBBB in the frontal plane, as indicated with the red arrows in figure 6A . The depolarization of the right ventricle will be normal and the impulse will travel from the right ventricle to the left ventricle via the apex. The vector starts pointing caudal (see figure 6B) changing in a vector with the main direction being cranial ( figure 6C and 6D). The last part of the VCG loop will be directed more to the left because the depolarization follows the left ventricular free wall (figure 6D). Looking in the horizontal plane, the VCG will mainly be pointing posterior towards the left ventricle, because the right ventricle depolarization is ahead of the left ventricular depolarization. 1.4.3 RBBB Figure 7 Schematic overview of depolarization with RBBB in the frontal plane, as indicated by the red arrows in A. Depolarization of the left ventricle will be ahead of depolarization of the left ventricle. The impulse will travels via the apex of the heart towards the right ventricle resulting in a mainly cranial direction (C en D). 9 In figure 7 a schematic overview of the depolarization in the frontal plane for a subject with RBBB is given. The red arrows in figure 7A indicate the frontal plane. The impulse will travel from the left ventricle to the right ventricle via the apex. One would expect again a mainly cranial direction of the loop (figure 7C and 7D). The vector will point to the right during the beginning of depolarization (figure 7B) and will swing to the left as depolarization in the left ventricle takes place. When the depolarization in the left ventricle is completed, the vector will turn to the right side again (figure 7D) The horizontal plane will indicate that the cardiac vector points anterior in this case, because the left ventricle will be depolarized before of the right ventricle. 1.4.4 Pacing Figure 8 Pacing in the horizontal plane (B and C) and in the frontal plane (E). The green arrows in A indicate the horizontal plane. The red arrows in D indicate the frontal plane. Lead placement will influence the direction of the cardiac electrical vector as is shown with the red arrows in B,C and E. A change in electrical activation will not only occur in case of bundle branch blocks, but the use of a pacemaker will also influence the electrical impulse propagation over the heart. The changes in the impulse conduction will be visible in the VCG as well. Generally speaking it is possible to pace the left or the right ventricle (LV or RV). The main difference in the VCG between LV and RV will be observed in the horizontal plane, as is shown in figure 8B. When pacing the right ventricle, the vector will be directed posterior. Pacing the left ventricle will lead to a vector directed anterior. To indicate vector directions on the right-left and the cranial-caudal axes, the exact placement of the lead will influence this direction. The position of the leads with respect to the X-axis will influence the right-left direction. A lead positioned to the right, will generally cause the vector to be directed to the left and vice versa (figure 8C). When positioning the lead towards the apex of the heart this will lead to a vector in cranial direction, while a pace position towards the basis of the heart will give a more caudal directed vector (figure 8E). 10 It is important to notice that RV pacing for a LBBB subject will most likely not change the directions of the vector drastically. The same characteristics will be visible because the pacing will cause the right ventricle to depolarize ahead of the left ventricle, as is the case with a LBBB. The same holds for LV pacing of a RBBB subject. 1.5 Project goal The goal of this project is to qualitatively describe vectorcardiograms for patients suffering from heart failure accompanied with conduction disorders that require CRT. The pathologic VCG’s will be compared to a normal VCG. Influence of lead placement will be investigated by analysis of vectorcardiograms after right and left ventricular pacing. 11 2. Materials and Methods 2.1 Patient population and data acquisition Standard 12-lead ECGs were recorded for patients with a LBBB, a RBBB, and for healthy individuals. The differentiation between patients with a LBBB and a RBBB was made according to the standard criteria on the 12-lead ECG. A bundle branch block can be diagnosed when the duration of the QRS complex on the ECG exceeds 120 milliseconds. A right bundle branch block typically causes prolongation of the last part of the QRS complex, and may shift the heart's electrical axis slightly to the right. Left bundle branch block widens the entire QRS complex, and in most cases shifts the heart's electrical axis to the left [2]. All patients with a LBBB and patients with a RBBB required CRT. For these latter groups the intrinsic rhythm was measured, before the pacemaker was activated. The pacemaker was then used for RV and LV pacing and the ECG was recorded again. For the healthy individuals only the intrinsic rhythm was recorded. During the implantation of the CRT device the 12-lead ECG was recorded for a period of 5 seconds for each setting (intrinsic, RV pacing, and LV pacing). Data was exported and analyzed offline. The data sampling rate was 977 points per second and the measured voltages were in mV. All procedures were performed in the Catharina Hospital in Eindhoven. 2.2 Signal analysis 2.2.1 Filtering of the ECG signal Pacemaker spikes and baseline wander in the ECG signal were removed by a Butterworth filter. Frequencies above 30 Hz and frequency below 0.25 Hz were eliminated. The lowpass filter had less than 3 dB of ripple in the passband, defined from 0 to 30 Hz, and at least 50 dB of attenuation in the stopband, which was defined from 60 Hz to 489 Hz (the Nyquist frequency). The response of the highpass filter was set to less than 3 dB of ripple in the passband, defined from 0.95 Hz to the Nyquist frequency at 489 Hz, and at least 50 dB of attenuation in the stopband from 0 Hz to 0.25 Hz. 2.2.2 Calculation of the VCG As described in paragraph 1.3, Frank used a special electrocardiographic lead system, consisting of seven electrodes, to derive the X, Y and Z leads [5]. To minimize clinical interference the standard recorded 12-lead ECG was used to calculate the Frank X, Y and Z leads, instead of changing to a Frank lead system. To calculate the X, Y and Z leads from a standard 12-lead ECG the inverse Dower matrix was used [6]. This method operates by calculating the VCG as a fixed linear combination of ECG signals as depicted below. ܺ = ሺ−0.172ܸଵ − 0.074ܸଶ + 0.122ܸଷ + 0.231ܸସ + 0.239ܸହ + 0.194ܸ + 0.156 ܫܮ− 0.010ܫܫܮሻሺ1ሻ ܻ = ሺ0.057ܸଵ − 0.019ܸଶ − 0.106ܸଷ − 0.022ܸସ + 0.041ܸହ + 0.048ܸ − 0.227 ܫܮ+ 0.887( )ܫܫܮ2) 12 ܼ = ሺ−0.229ܸଵ − 0.310ܸଶ − 0.246ܸଷ − 0.063ܸସ + 0.055ܸହ + 0.108ܸ + 0.022 ܫܮ+ 0.102ܫܫܮሻ (3) Herein V1 to V6 indicate the precordial leads and LI and LII the standard bipolar leads I and II. One of the main problems with this method is that the measurement is very dependent of the placement of the electrodes and is not adapted for patients with different body compositions and geometries. Compared to other proposed methods for the synthesis of a VCG this method gives the best results with a minimum of interference with clinical practice [7] . 2.2.3 Definition of an average heartbeat The ECG signal was recorded for approximately 5 seconds, to find a periodic electrical rhythm consisting of a number of 5 to 10 heartbeats without any premature ventricular contractions. For every separate heartbeat the electrical activation will be similar. To enhance the signal to noise ratio one average heartbeat was calculated, which contains the same characteristics of the separate heartbeats. Plotting several heartbeats apart will not give any additional characteristics. The first step to differentiate between separate heartbeats was to calculate the magnitude of the cardiac vector (Vc) at each instant using equation 4: ||ܸ ሺ݅ሻ|| = ඥܺሺ݅ሻଶ + ܻሺ݅ሻଶ + ܼሺ݅ሻଶ (4) With X (i), Y (i) and Z (i) the calculated Frank leads at the ith sample point. Local maxima were then detected which corresponded with the depolarization peak, or the R-wave, during the heart cycle. The part of the signal between two peaks therefore corresponds with one single heartbeat. To normalize the separate heartbeats, each heartbeat was resampled to 1000 sample points. An average heartbeat was calculated by taking the mean of all differentiated heartbeats in the signal. 2.2.4 Differentiation of the depolarization wave Because CRT aims to resynchronize the contraction of both ventricles, the focus of the description of the VCG lies in the depolarization loop, since depolarization precedes the ventricular contraction. To be able to differentiate between depolarization and repolarization waves in the averaged heartbeat, the beginning and the end of depolarization wave need to be defined. Figure 9 shows the steps to differentiate the depolarization wave. First a global representation of the magnitude of the cardiac vector was obtained by smoothing of the signal with a lowpass filter, which has less than 3 dB of ripple in the passband, defined from 0 to 8 Hz, and at least 50 dB of attenuation in the stopband, which is defined from 30 Hz to the Nyquist frequency. Starting from sample point 1 in the smoothed signal (represented by the red signal in figure 9A), the first minimum of the time derivative was found (indicated by w1 in figure 9A). 13 Subsequently, the next maximum of the time derivative was found (indicated by w2 in figure 9A). These two points set the window for finding the minimum in the unfiltered signal (the green signal in figure 9A and 9B), which represents the end of depolarization. To find the beginning of depolarization, first the minimum between sample points 700 and 1000 of the filtered signal was used as a reference point (indicated by point w3). Then the maximum value of the time derivative of the filtered signal following the minimum, is used as a second reference point (indicated by point w4 in figure 9A). A window was created again between the two reference points and the minimum value in the unfiltered signal was set as the beginning of depolarization. Figure 9B shows the same representation of the magnitude of the cardiac vector (the green signal from signal 9A), with the marked points for the depolarization wave. Following the global maximum in the filtered signal after the end of depolarization (point p1 in figure 9A), the first point in the time derivative of the filtered signal with a positive value was found (point p2 in figure 9B). The signal is shifted to set point p2 as the start of the heartbeat. Figure 9. Example of detection of the depolarization wave. The unfiltered signal is plotted in green (panel A and B), the filtered signal in red (panel A). In panel A, the point indicated with w1 represents the first minimum in the time derivative of the red signal and w2 indicates the following maximum in the time derivative of this signal. Point w3 is found to be the minimum of the filtered signal for the depolarization peak and point w4 indicated the maximum in the time derivative of the filtered signal. The black dots indicate the beginning and end of the depolarization wave. Points p1 and p2 (in panel A) are used to shift the signal as shown in panel B. 14 2.3 Qualitative measures of the VCG To qualitatively describe the VCG, the depolarization loop was plotted in a 3D representation and in three 2D representations (the frontal plane, the sagittal plane and the horizontal plane). As mentioned in paragraph 1.3, axes defined for the Frank leads are represented by a right-left axis (X), a head-to-feet (cranial to caudal) axis (Y) and a front-back (anteriorposterior) axis (Z). In figure 10 the frontal plane (indicated by the blue arrows), the sagittal plane (indicated by the red arrows) and the horizontal plane (indicated by the green arrows) are shown, in correspondence with the planes defined in paragraph 1.4. With the use of the directional terms defined in each plane, it is possible to qualitatively describe the movement of the cardiac vector in time. Figure 10 3D representation and definition of the 2D planes. The frontal plane is indicated by the red arrows, the sagittal plane is indicated by the blue arrows and the horizontal plane is visualized with the green arrows. Directional terms are defined in each plane to allow for a qualitative description of the movement of the cardiac vector in time. 2.4 Quantitative measures of the VCG 2.4.1 Mean electrical axis To help analyzing the VCG loops qualitatively, some parameters are calculated to quantify changes in direction and magnitude of the cardiac electrical vector. The maximal depolarization vectors in 3D and in the 2D planes are found by calculating the magnitude of the vectors at the top of the depolarization wave (as defined in figure 9). Using geometry formulas (the inverse tangent) in the 2D planes, the angle of the maximal vector was calculated. The calculated angles were defined as in figure 11. Figure 11 Angle definitions for the 2D planes 15 To diagnose bundle branch blocks from the 12-lead ECG, not only the duration of the QRS complex but normally also the mean electrical axis is calculated. It is defined as the preponderant direction of the potential during depolarization. Changes in the conduction pathway of the impulse are thus visible by looking at this mean electrical axis. For the 2D projections the mean electrical axis is calculated and plotted in the figure. The axis can be calculated by summation of the maximal value and the minimum value for the X, Y and Z leads [2]. A mean electrical axis was calculated in the 2D planes according to the definitions in figure 11. 2.4.2 VCG loop area The area covered by the VCG loop in the different quadrants in the 2D planes is calculated as well. As shown in figure 12, a grid of pixels is set up on an area with known dimensions. The number of pixels inside the loop is counted and divided by the total number of pixels on the grid. Because the dimensions are known, the area covered by the loop could be calculated now. The four quadrants in every plane are defined as in figure 10. The quadrant with the maximum area will indicate the preponderant direction of the VCG loop. 2.4.3 Duration of depolarization From a standard 12-lead ECG it is possible to find the width of the depolarization wave. With the use of begin- and endpoint of the depolarization wave (as found in figure 9), the duration of the depolarization loop was calculated. Figure 12 Schematic drawing of an example for area calculation in quadrant three. The total number of pixels is 100 on a total area of 2 1mV . 23 pixels inside the loop are colored 2 black, indicating a loop area of 0.23 mV 16 3. Results 3.1 Measurements Based on available subjects and the availability of the heart catheterization room, the ECG’s of one healthy subject and two subjects with a RBBB (RBBB1 and RBBB2 respectively) and two subjects with a LBBB (LBBB1 and LBBB2 respectively) were recorded. All four BBB subjects were paced from the right and the left ventricle, but the exact pacing location was unknown. VCG loops of all measurements were calculated and analysed. 3.2 Healthy subject, LBBB subjects and RBBB subjects 3.2.1 Healthy subject Figure 13 shows the 3D representation of the VCG loop for the healthy subject. To visualize a timescale in the VCG loop, all depolarization loops are plotted in red and green, switching colour every 10 milliseconds. The projection of the loop in the frontal, sagittal and horizontal plane is also visible in grey, with arrows indicating the followed direction in all figures. An increased loop length for the separate red and green parts corresponds with a higher speed of conduction of the electrical impulse. Figure 13 3D plot of VCG loop for the healthy subject. Depolarization loop is colored red and green every 10 ms. Projections on the 2D planes are shown in grey. The axes are in mV. The preponderant direction of the loop is caudial, posterior and to the left. Figure 14 gives the projections of the same loop of the healthy subject in the frontal and the horizontal plane. The quantitative measures are printed below the loops. In the frontal plane the vector starts with a cranial and right deflection and moves towards a left and caudial direction. The loop is closed with a vector pointing in the cranial and right direction again. The maximal depolarization vector, the mean electrical axis and the maximal area in the quadrant are all found in the caudal and left quadrant. 17 Figure 14 2D Representation of the VCG loop in the frontal and horizontal plane for a healthy subject. Coloring of the depolarization loop is in correspondence with the 3D plot. The bold black vector with the closed tail is the maximal depolarization vector and the thin speckled vector that is plotted corresponds with the mean electrical axis. Axes are in mV. Figures and measures indicate a mainly caudal, posterior and left direction in correspondence with figure 13. In the horizontal plane the depolarization starts in the anterior and right direction. The vector moves towards the posterior and left quadrant. Quantitative measures all indicate a preponderant direction in this quadrant. The loop ends with a small posterior and right vector. 3.2.2 Bundle branch blocks In figure 15 the 3D representations of the VCG loop for the subject LBBB1 and for subject RBBB1 are shown. As compared to the healthy subject the depolarization time is longer, indicating a lower speed of conduction of the electrical impulse. For the RBBB1 subject, as compared to the LBBB1 subject, the speed of conduction is higher, as indicated by the shorter depolarization time and the increased length for the separate red and green loop parts. Figure 15 3D plots of the VCG loop for the LBBB1 and the RBBB1 subject. Projections of the 2D planes are visible in grey, with arrows indicating the followed path. Axes are in mV. The preponderant direction of the loop for the LBBB1 is posterior, while the loop of the RBBB1 subject is mainly pointing anterior. 18 From both loops in figure 15, it is clear that for the LBBB1 the vector is pointing posterior, while for the RBBB1 subject this is mainly in the anterior direction. To describe the similarities and differences between the two loops, figure 16 shows the 2D representations of the VCG loop of both subjects in the frontal and the horizontal plane. Figure 16 Frontal and horizontal plane representation of the VCG loop of the LBBB1 and RBBB1 subjects. The maximal vector and mean electrical axes are plotted in the figures as described with figure 14. Axes are in mV. The main difference between the loops can be found in the horizontal plane, while in the frontal plane the loops look very similar. For both subjects the loop in the frontal plane is very similar. A clearly cranial direction of the vector is seen, moving from the left to the right. For both subjects the preponderant direction is to the right, as the calculated areas indicate. The main difference between both loops can be found in the horizontal plane. The maximal vector, the mean electrical axes and the calculated area indicate a posterior direction for the LBBB1 subject, while a anterior direction is seen for the RBBB1 subject. 19 3.2.3 Overall observations In the frontal plane the caudal direction for a healthy subject is opposite to the observed cranial direction for the BBB subjects. Another difference can be found in the mainly right direction for the BBB subject, as compared to the left direction for the healthy subject. All is summarized in table 1. INTRINSIC RHYTHM Depolarization time in ms Angle max. vector in degrees (frontal/sagittal/horizontal) Angle mean electrical axis in degrees (frontal/sagittal/horizontal) Quadrant with max. area (frontal) Quadrant with max. area (sagittal) Quadrant with max. area (horizontal) Healthy 112 50/43/ -54 LBBB1 196 -88/-55/ -103 LBBB2 143 -121/-9/ -93 RBBB1 141 -83/-141/ 105 RBBB2 208 -114/-107/ 137 59/47/ -58 -100/-39/ -98 -126/-18/ -103 -100/-133/ 101 -102/-112/ 117 CaudalLeft PosteriorCaudal PosteriorLeft CranialRight PosteriorCranial PosteriorRight CranialRight PosteriorCranial PosteriorRight CranialRight AnteriorCranial AnteriorRight CranialRight AnteriorCranial AnteriorRight Table 1 Summary of quantitative measurements for the healthy and the BBB subjects. The difference between a healthy subject and the BBB subject is found in the caudal and left direction. The main difference between the LBBB and RBBB subjects is found in the posterior and anterior directions. 3.3 RV and LV pacing 3.3.1 RV pacing In figure 17 the 2D representations of the VCG loop after RV pacing for the LBBB1 and RBBB1 subject are shown. Figure 17 2D Representation of the VCG loop of the LBBB1 and RBBB1 subject in the sagittal plane after RV pacing. The maximal vector and mean electrical axes are plotted in the figures as described with figure 14. Axes are in mV. Main difference between the loops can be found in the cranial direction for LBBB1 and the caudal direction for RBBB1. Both loops show a posterior direction. 20 A mainly posterior direction, which is found for all four BBB subjects, is observed. A difference is seen in the cranial direction for LBBB1 and a caudal direction for RBBB1. All quantitative measures are summarized in table 2 for the RV pacing. The table shows a mainly right and posterior direction for all subjects. As was visible in figure 17, a difference is found in the cranial direction for the LBBB subjects and a caudal direction for the RBBB subjects. RV PACING Depolarization time in ms Angle max. vector in degrees (frontal/sagittal/horizontal) Angle mean electrical axis in degrees (frontal/sagittal/horizontal) Quadrant with max. area (frontal) Quadrant with max. area (sagittal) Quadrant with max. area (horizontal) LBBB1 203 -110/-71/ -138 LBBB2 170 -111/-55/ -119 RBBB1 147 127/18/ -103 RBBB2 192 136/17/ -109 -115/-75/ -150 -111/-59/ -123 136/11/ -102 139/14/ -106 CranialRight PosteriorCranial PosteriorRight CranialRight PosteriorCranial PosteriorRight CaudalRight PosteriorCaudal PosteriorRight CaudalRight PosteriorCaudal PosteriorRight Table 2 Summary of quantitative measures after RV pacing for the four BBB subjects. Similarities are found in the posterior direction for all subjects. Main difference is found between LBBB subject and RBBB subjects in the cranial and caudal directions. 3.3.2 LV pacing For the LV pacing the 2D representations of the VCG loop of LBBB1 and RBBB1 in the frontal plane are shown in figure 18. A mainly left direction for the LBBB1 subject is observed, while for the RBBB1 subject this main direction is to the right. Figure 18 2D Representations of the VCG loops in the frontal plane for subjects LBBB1 and RBBB1 after LV pacing. The maximal vector and mean electrical axes are plotted in the figures as described with figure 14. Axes are in mV. The plots in the frontal plane indicate a difference in the right and left direction. For LBBB1 the loop is mainly in the cranial/left quadrant, while for the RBBB1 subject the loop is mainly in the cranial/right quadrant. 21 For both 2D projections of the VCG loop from figure 18, a cranial direction is observed and calculated from the quantitative measures. All quantitative measures are summarized in table 3 for the LV pacing. The table gives a preponderant anterior and cranial direction. A difference is found between the left direction for both LBBB subject and a right direction for both RBBB subjects. LV PACING Depolarization time in ms Angle max. vector in degrees (frontal/sagittal/horizontal) Angle mean electrical axis in degrees (frontal/sagittal/horizontal) Quadrant with max. area (frontal) Quadrant with max. area (sagittal) Quadrant with max. area (horizontal) LBBB1 159 -41/-159/ 63 LBBB2 178 -7/-175/ 63 RBBB1 214 -131/-160/ 79 RBBB2 213 -1/-171/ 80 -39/-165/ 71 -46/-165/ 75 -102/-157/ 96 -32/-172/ 78 CranialLeft AnteriorCranial AnteriorLeft CranialLeft AnteriorCranial AnteriorLeft CranialRight AnteriorCranial AnteriorRight CranialRight AnteriorCranial AnteriorRight Table 3 Summary of quantitative measures after LV pacing for the four BBB subjects. Similarities are found in the anterior direction for all subjects. Main difference is found between LBBB subject and RBBB subjects in the left and right directions. 3.3.3 Overall observations RV pacing results in a cardiac vector directed posterior for all BBB subjects, while for LV pacing an anterior vector is observed for all BBB subjects. When the LBBB subjects are paced from the right ventricle, the direction of the VCG loop does not change with respect to the intrinsic rhythm. RV pacing does influence the VCG loop direction for the RBBB subjects, as can be observed in table 1 and table 2. The VCG loop is directed posterior and caudal after pacing, while the loop was intrinisically directed anterior and cranial. For the RBBB subjects pacing from the left ventricle does not influence the main direction of th VCG loop. For the LBBB subjects, when comparing table 1 and table 3, the VCG loop is directed anterior and left after LV pacing, with an intrinsic posterior and right directed loop. 22 4. Discussion and Conclusions 4.1 Healthy VCG In correspondence with the theory described in paragraph 1.4, the results show a VCG loop for the healthy subject which is directed mainly to the left, caudal and posterior. However, at the end of depolarization, a cranial and right pointing vector is observed, which is somewhat unexpected. This deviation might lie within the normal limits and could be explained by a slightly different orientation of the heart in the thorax. A rotation to the front around the right-left axis could explain the unexpected projection in the frontal plane in this case. Increasing the number of normal VCG’s will give more insight in this issue. 4.2 VCG of LBBB intrinsic and with pacing The VCG loops of the subjects with an intrinsic LBBB rhythm are directed mainly right, cranial and posterior, as was expected. RV pacing does not influence the direction of the VCG loop, because it resembles the electrical impulse pathway for the intrinsic LBBB rhythm. In both cases the right ventricle depolarizes ahead of the left ventricle. LV pacing influences the direction of the VCG loop by changing the vector to a mainly anterior and left direction. 4.3 VCG of RBBB intrinsic and with pacing For the intrinsic rhythm of the RBBB subjects an expected, mainly right, cranial and anterior directed VCG loop, is observed. In this case LV pacing resembles the impulse pathway for the intrinsic RBBB rhythm, while RV pacing changes the direction of the VCG loop. After RV pacing the vector is directed mainly caudal and posterior. 4.4 Pacing lead position As mentioned in paragraph 3.1, the exact lead positions, for both RV and LV pacing, were unknown. It seems reasonable to conclude that this exact lead position determines the main direction of the VCG loop, as differences were found between RV and LV pacing measurements mutually. In figure 19 two different lead positions are schematically shown for LV pacing (in red and green). The direction to the left for the RBBB subjects is explained by a position of the pacemaker towards the septum (shown in red in figure 19). The heart muscle mass at the left side of the Figure 19 Schematic view on the lead positions for LV pacing in a section perpendicular to the septum of the heart. The red dot indicated the pacing position for the RBBB subjects, with the red line indicating an excess of muscle mass at the left side of the lead, resulting in a left oriented vector. The green dot indicates the pacing position for the LBBB subjects, with the green line indicating an excess of muscle mass at the right side of the lead, resulting in a right oriented vector. 23 pacemakers exceeds the muscle mass at the right side of the pacemaker. Therefore the vector will be directed to the left first. For the LBBB subjects the lead was positioned more to the left (shown in green in figure 19), resulting in a mainly right directed vector. This principle holds for all planes or directions, but also for RV lead placement. This means that it is possible to extract the exact lead position from the VCG. It is necessary to monitor the exact lead positions in future, to get more data on lead placement and differences in VCG loop directions. 4.5 Normalization of the VCG In chapter 2 it was mentioned that the method chosen to calculate the X, Y and Z leads, with the use of the inverse Dower matrix, is very dependent of the placement of the electrodes and is not adapted for subjects with different body compositions and geometries. In literature this method is described as the preferred method for VCG synthesis. For now, this method is appropriate to focus on patterns visible in the VCG and the minimal interference with clinical practice is a great benefit. To compensate for different geometries, a change of basis of the coordinate system can help to normalize the VCG loops. MRI images could provide information about the actual anatomy and orientation of the heart. When the anatomy and orientation of the heart are known, a coordinate system with one axis along with the heart axis (from basis to apex) can be used for all subjects. To change the basis of the coordinate system a 3D rotation matrix (R), using Euler angles, can be used: ݏܥሺߙሻݏܥሺߛሻ − ݏܥሺߚሻܵ݅݊ሺߙሻܵ݅݊ሺߛሻ ݏܥሺߛሻܵ݅݊ሺߙሻ + ݏܥሺߙሻݏܥሺߚሻܵ݅݊ሺߛሻ ܵ݅݊ሺߚ_ܵ݅݊ሺߛሻ R= ቌݏܥሺߚሻݏܥሺߛሻܵ݅݊ሺߙሻ − ݏܥሺߙሻܵ݅݊ሺߛሻ ݏܥሺߙሻݏܥሺߚሻݏܥሺߛሻ − ܵ݅݊ሺߙሻܵ݅݊ሺߛሻ ݏܥሺߛሻܵ݅݊ሺߚሻቍ ܵ݅݊ሺߙሻܵ݅݊ሺߚሻ −ݏܥሺߙሻܵ݅݊ሺߚሻ ݏܥሺߚሻ With α, β and γ corresponding to the Euler angles. The rotation by angle α represents the rotation around the axis ‘z’ of the Cartesian coordinate system or the angle between the ‘x’ axis and the line of nodes (N), which is shown in figure 20. N is defined by the intersection between the ‘xy’ and ‘DE’ coordinate planes. β gives the rotation between the original ‘z’ axis and the new ‘F’ axis. Finally the γ rotation corresponds with the rotation around the ‘F’ axis of the new reference frame or, as figure 20 shows, the rotation between the line of nodes and the new ‘D’ axis. 4.6 VCG in CRT In CRT, a biventricular pacemaker is used, which enables activation of the myocardium at different locations and with different time delays. Compared to the RV and LV pacing only, this will give a more Figure 20 Change of basis using Euler angles (α, β en γ). The line of nodes (N) is defined at the intersection between the 'xy' and 'DE' coordinate planes. The blue coordinate system is the initial xyz coordinate system. The red coordinate system (DEF) gives the result after rotation. 24 complicate interpretation of the VCG loop. More research is needed to investigate the influence of such a biventricular device. A database of VCG characteristics with different lead positions and different timing sequences should be set up, by conducting measurements during CRT procedures. Correlation with optimal dP/dt measurements could eventually help to find the optimal lead position and timing sequence, based on VCG loops. 4.7 Quantification of the VCG Besides the qualitative description of the VCG loops, quantification of VCG loops can help to find objective measures for optimal electrical conduction pathways. At this moment, the number of measurements is too low to obtain any conclusions about quantitative measures. The loop area for different quadrants was calculated to help with the qualitative description of the VCG loops. The total depolarization loop area can be used as a quantitative measure. An increased are under the depolarization loop will correspond with an increased depolarized muscle mass and an increased R-wave in the 12-lead ECG. Besides the loop area, the loop length can be calculated by summing the magnitude of the vectors between two consecutive sample points. An increased loop length, with a short depolarization time will give an indication about the average speed of conduction of the electrical impulse. An increased speed of conduction will result in a more synchronous contraction. The ratio between the loop area and the loop length will give information about the morphology of the loop. It should be investigated which quantitative measures can help with the interpretation of the VCG loops. 4.8 Repolarization For now, the focus was on the description of the depolarization loop. Differentiation and description of the repolarization loop can also give useful information. For the BBB subjects the normal pathway for the impulse conduction is disturbed and the repolarization will start in a part of the heart before depolarization is completed. Therefore, the direction of the repolarization and the depolarization loops will be contrary for the BBB subjects, while for the healthy subject the repolarization and depolarization loop are directed more parallel. More research is needed to describe and analyze the repolarization loops. 4.9 Summary of conclusions Because the direction of the VCG loop is a reflection of the electrical impulse pathway, the presented VCG loops give an intuitive insight into the conduction of the electrical impulse over the heart. Differences in the impulse conduction for subjects with a BBB as compared to a healthy subject and as compared for LBBB and RBBB subjects apart, are found. Although the number of measurements is low, it should be noticed that the same patterns are visible 25 for homologous subjects. Differences between RV pacing and LV pacing were found and information about the lead position can be extracted from the VCG loops. 26 5. Future Directions As mentioned already in paragraph 4.5, MRI images should be produced preceding a CRT procedure, to indicate the orientation of the heart in the thorax. The MRI images can be used to rotate the coordinate system, to a system with one axis along with the septum. The plotted VCG loops will be indifferent with respect to heart orientation, which leads to a normalization of the VCG loops. Measurements were recorded now during CRT procedures and analyzed offline afterwards. In future, real time visualization of the VCG loops during the procedure will make it easier to make use of the interpretation of the VCG for CRT. Some first steps are already made in this direction. Furthermore, it should be investigated whether a finite element model of the cardiac electro mechanics could help with the prediction of the VCG loops in more detail. In 2008 A. Lenssen developed a model which could help to produce the 12-lead ECG signals [8]. The model can help to simulate the influence of the lead positions and conduction blocks at different regions of the heart, but also the influence of body composition and different geometries of the heart. The model should be improved and can be used to develop VCG loops. Theoretical insight into the influence on the VCG loop can help to improve insight in practice. Increasing the number of measurement, for healthy subjects and for BBB subjects, will help to get insight into the quantitative measures of the VCG loops and it will help to develop a database for the qualitative description of the VCG loop. For the RV and LV pacing measurements the lead position should be monitored and varied over the ventricles. This will give more data concerning the position of the pacemaker and the change in direction of the VCG loop. Also pressure data should be assembled to find correlation between dP/dt measurements and VCG loops. 27 6. References 1. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008, The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM), Eur Heart J (2008) doi 10.1093/eurheartj/ehn309, September 17th 2008 2. Textbook of Medical Physiology Eleventh Edition, Arthur C. Guyton and John E. Hall, 2006 3. Optimization of cardiac resynchronization therapy: addressing the problem of “nonresponders”, by D.J. Fox, A.P. Fitzpatrick and N.C. Davidson, Heart, 2005;91;10001002 4. Application of LVdP/dtMAX in the optimization of cardiac resynchronization therapy, Berry M. van Gelder, Ph.D. , Department of Electrophysiology , Catharina Hospital Eindhoven, April 2009 5. An Accurate, Clinically Practical System For Spatial Vectorcardiography, Ernest Frank, Circulation 1956;13;737-749 6. On deriving the electrocardiogram from vectorcardiographic leads, by G.E. Dower and H.B. Machado, Clin. Cardiology, 1980;3:87 7. Vectorcardiogram Synthesized From a 12-lead ECG: Superiority of the Inverse Dower Matrix, by Lars Edenbrandt and Olle Pahlm, Journal of Electrocardiology, 1988;21(4); 361-367 8. From cardiac electrical activity to the ECG (a finite element model), A.M.J. Lenssen, Master Thesis, Eindhoven University of Technology, 2008 28 Appendix A – ECG’s Healthy Subject LBBB1 Intrinsic 29 LBBB2 Intrinsic RBBB1 Intrinsic 30 RBBB2 Intrinsic LBBB1 RV Pacing 31 LBBB2 RV Pacing RBBB1 RV Pacing 32 RBBB2 RV Pacing LBBB1 LV Pacing 33 LBBB2 LV Pacing RBBB1 LV Pacing 34 RBBB2 LV Pacing 35 Appendix B – 3D Representations of VCG loops Healthy Subject LBBB1 Intrinsic 36 LBBB2 Intrinsic RBBB1 Intrinsic 37 RBBB2 Intrinsic LBBB1 RV Pacing 38 LBBB2 RV Pacing RBBB1 RV Pacing 39 RBBB2 RV Pacing LBBB1 LV Pacing 40 LBBB2 LV Pacing RBBB1 LV Pacing 41 RBBB2 LV Pacing 42 Appendix C – 2D Representations of VCG loops Healthy Subject LBBB1 Intrinsic 43 LBBB2 Intrinsic RBBB1 Intrinsic 44 RBBB2 Intrinsic LBBB1 RV Pacing 45 LBBB2 RV Pacing RBBB1 RV Pacing 46 RBBB2 RV Pacing LBBB1 LV Pacing 47 LBBB2 LV Pacing RBBB1 LV Pacing 48 RBBB2 LV Pacing 49