* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Euprymna scolopes -Vibrio fischeri Symbiosis: A Biomedical
Survey
Document related concepts
Transcript
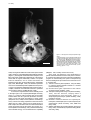
J. Molec. Microbiol. Biotechnol. (1999) 1(1): 13-21. The Squid-Vibrio Symbiosis as a Biomedical Model 13 JMMB Symposium The Euprymna scolopes -Vibrio fischeri Symbiosis: A Biomedical Model for the Study of Bacterial Colonization of Animal Tissue Edward G. Ruby* Pacific Biomedical Research Center University of Hawaii, Manoa, Hawaii Abstract The diversity of microorganisms found in the marine environment reflects the immense size, range of physical conditions and energy sources, and evolutionary age of the sea. Because associations with living animal tissue are an important and ancient part of the ecology of many microorganisms, it is not surprising that the study of marine symbioses (including both cooperative and pathogenic interactions) has produced numerous discoveries of biotechnological and biomedical significance. The association between the bioluminescent bacterium Vibrio fischeri and the sepiolid squid Euprymna scolopes has emerged as a productive model system for the investigation of the mechanisms by which cooperative bacteria initiate colonization of specific host tissues. The results of the last decade of research on this system have begun to reveal surprising similarities between this association and the pathogenic associations of disease-causing Vibrio species, including those of interest to human health and aquaculture. Studies of the biochemical and molecular events underlying the development of the squid-vibrio symbiosis can be expected to continue to increase our understanding of the factors controlling both benign and pathogenic bacterial associations. Introduction Two areas of biomedical significance have emerged during the past 20 years. The first of these is the rise in coastal areas across the world of bacterial diseases borne by marine and brackish-water bacteria in the genus Vibrio (Blake, 1983; Colwell, 1996). The second is the growing awareness of the importance of cooperative bacterial colonization of animal tissues to the proper development and health of the host (McFall-Ngai, 1998a; Rook and Stanford, 1998). Progress in addressing questions in both of these areas has been made through the investigation of a symbiotic association involving Euprymna scolopes, a shallow-water squid, and Vibrio fischeri, a bioluminescence bacterium. This association has developed into a paradigm not only for the study of symbiosis in the marine environment, but for the myriad of both cooperative and pathogenic bacteria-animal host interactions that characterize the *For correspondence. Email [email protected]; Tel. (808) 539-7309; Fax. (808) 599-4817. © 1999 Horizon Scientific Press biological world. The principal interactions between bacteria and animal tissue occur along the apical surfaces of host epithelial cells. Typically, a diverse consortium of microorganisms and host cells forms a dynamic, cooperating unit that helps maintain a healthy microenvironment within the colonized tissues. Such associations are ubiquitous, exhibit species specificity and, with few exceptions (Breznak and Brune, 1994; Whittaker et al., 1996; Hooper et al., 1998), are poorly understood. A better understanding of the process by which bacteria and host cells exchange the signals that underlie these phenomena is an important research goal of biomedical research. Over the past decade the E. scolopesV. fischeri association, a natural and experimentally facile model system, has been used to discover the nature of the mechanisms by which animals form beneficial associations with microorganisms, and to determine how these mechanisms are related to, but have diverged from, those that function in pathogenic infections. Initial studies concentrated on a description of the system, leading to an understanding of the light organ morphology in both the juvenile (Montgomery and McFall-Ngai, 1993; Weis et al., 1993; Hanlon, et al., 1997) and the adult (McFall-Ngai and Montgomery, 1990; Montgomery and McFall-Ngai, 1998) animal. Similarly, the pattern of growth of V. fischeri cells during the initiation of the association (Ruby and Asato, 1993), and the role of the bacteria in the induction of its subsequent development (Foster and McFall-Ngai, 1998; McFall-Ngai and Ruby, 1998) were described. In addition, unexpected phenomena were discovered, such as the pronounced and dynamic diel rhythm dominating the state of the association (Lee and Ruby, 1994a; Boettcher et al., 1996), and the presence of the antibacterial enzyme haloperoxidase within the contents of the light organ crypts (Weis et al., 1993). This review describes the colonization of the squid light organ by V. fischeri , emphasizing those bacterial determinants that have homologs or analogs among pathogenic Vibrio species. What we have learned about the development and specificity of this process by an examination of the host-imposed obstacles or barriers to colonization is also outlined. The review concludes with a discussion of the possible roles current and future studies of the E. scolopes-V. fischeri association may play in biomedically important areas of research. The Symbiotic Partners Euprymna scolopes is a small, nocturnal, sepiolid squid (Berry, 1912) that is bioluminescent owing to the presence of bacterial symbionts contained within a complex, bilobed, light-emitting organ (Nesis, 1987; McFall-Ngai and Montgomery, 1990). Light generated by these bacteria allows the host squid to produce a controlled, diffuse, ventral glow as an antipredatory behavior (McFall-Ngai, Further Reading Caister Academic Press is a leading academic publisher of advanced texts in microbiology, molecular biology and medical research. Full details of all our publications at caister.com • MALDI-TOF Mass Spectrometry in Microbiology Edited by: M Kostrzewa, S Schubert (2016) www.caister.com/malditof • Aspergillus and Penicillium in the Post-genomic Era Edited by: RP Vries, IB Gelber, MR Andersen (2016) www.caister.com/aspergillus2 • The Bacteriocins: Current Knowledge and Future Prospects Edited by: RL Dorit, SM Roy, MA Riley (2016) www.caister.com/bacteriocins • Omics in Plant Disease Resistance Edited by: V Bhadauria (2016) www.caister.com/opdr • Acidophiles: Life in Extremely Acidic Environments Edited by: R Quatrini, DB Johnson (2016) www.caister.com/acidophiles • Climate Change and Microbial Ecology: Current Research and Future Trends Edited by: J Marxsen (2016) www.caister.com/climate • Biofilms in Bioremediation: Current Research and Emerging Technologies Edited by: G Lear (2016) www.caister.com/biorem • Flow Cytometry in Microbiology: Technology and Applications Edited by: MG Wilkinson (2015) www.caister.com/flow • Microalgae: Current Research and Applications • Probiotics and Prebiotics: Current Research and Future Trends Edited by: MN Tsaloglou (2016) www.caister.com/microalgae Edited by: K Venema, AP Carmo (2015) www.caister.com/probiotics • Gas Plasma Sterilization in Microbiology: Theory, Applications, Pitfalls and New Perspectives Edited by: H Shintani, A Sakudo (2016) www.caister.com/gasplasma Edited by: BP Chadwick (2015) www.caister.com/epigenetics2015 • Virus Evolution: Current Research and Future Directions Edited by: SC Weaver, M Denison, M Roossinck, et al. (2016) www.caister.com/virusevol • Arboviruses: Molecular Biology, Evolution and Control Edited by: N Vasilakis, DJ Gubler (2016) www.caister.com/arbo Edited by: WD Picking, WL Picking (2016) www.caister.com/shigella Edited by: S Mahalingam, L Herrero, B Herring (2016) www.caister.com/alpha • Thermophilic Microorganisms Edited by: F Li (2015) www.caister.com/thermophile Biotechnological Applications Edited by: A Burkovski (2015) www.caister.com/cory2 • Advanced Vaccine Research Methods for the Decade of Vaccines • Antifungals: From Genomics to Resistance and the Development of Novel • Aquatic Biofilms: Ecology, Water Quality and Wastewater • Alphaviruses: Current Biology • Corynebacterium glutamicum: From Systems Biology to Edited by: F Bagnoli, R Rappuoli (2015) www.caister.com/vaccines • Shigella: Molecular and Cellular Biology Treatment Edited by: AM Romaní, H Guasch, MD Balaguer (2016) www.caister.com/aquaticbiofilms • Epigenetics: Current Research and Emerging Trends Agents Edited by: AT Coste, P Vandeputte (2015) www.caister.com/antifungals • Bacteria-Plant Interactions: Advanced Research and Future Trends Edited by: J Murillo, BA Vinatzer, RW Jackson, et al. (2015) www.caister.com/bacteria-plant • Aeromonas Edited by: J Graf (2015) www.caister.com/aeromonas • Antibiotics: Current Innovations and Future Trends Edited by: S Sánchez, AL Demain (2015) www.caister.com/antibiotics • Leishmania: Current Biology and Control Edited by: S Adak, R Datta (2015) www.caister.com/leish2 • Acanthamoeba: Biology and Pathogenesis (2nd edition) Author: NA Khan (2015) www.caister.com/acanthamoeba2 • Microarrays: Current Technology, Innovations and Applications Edited by: Z He (2014) www.caister.com/microarrays2 • Metagenomics of the Microbial Nitrogen Cycle: Theory, Methods and Applications Edited by: D Marco (2014) www.caister.com/n2 Order from caister.com/order 14 Ruby e } lo * m g 1990). The organ lies within the center of the squid’s mantle cavity, where it is continually bathed with ambient seawater (Figure 1). Histological and ultrastructural analyses of the light organ have revealed an integrated set of tissues that serves both to support nutritionally a culture of a specific luminous bacterium, and to control and direct the bioluminescence they produce (Montgomery and McFallNgai, 1992; McFall-Ngai, 1994). The bacterial symbionts are housed extracellularly within epithelium-lined spaces, or crypts. The polarized epithelium bears a dense layer of complex, branched microvilli within which the symbionts are invested (Lamarcq and McFall-Ngai, 1998). V. fischeri, the only bacterium found to naturally persist in the light organ of E. scolopes (McFall-Ngai and Ruby, 1991), is a common marine microbe that can be easily isolated either from this symbiosis (Boettcher and Ruby, 1990) or from light organ associations of other squids (Fidopiastis et al., 1998) or fishes (Nealson and Hastings, 1991), and from free-living populations in the environment (Ruby and Lee, 1998), and that can be readily grown on both complex and minimal media. An extensive literature exists concerning both the physiology and ecology of V. fischeri (Nealson and Hastings, 1991), and the biochemistry and molecular biology of its bioluminescence capability Figure 1. The Euprymna scolopes Symbiotic Light Organ A ventral dissection of an anesthetized adult animal reveals the bilobed light organ (lo) in the center of the mantle cavity (McFall-Ngai and Montgomery, 1990), and partially occluded by the ink sac (black). Each lateral face of the adult light organ has a single pore through which the bacteria-containing crypts, deep in the center of the lobes of the organ (*), are connected to the outside environment (e, eye; m, mantle; g, gill; bar = 1 cm). (Meighen, 1991; Dunlap, review in this issue). Since 1996, new advances in the development of genetic and biochemical tools for the study of the V. fischeriE. scolopes association have been important in facilitating progress in understanding this system. Principal among these bacterial genetic procedures and constructs for use in V. fischeri are: (i) Transfer of DNA by electroporation, using the ability to overcome innate restriction barriers in V. fischeri (Visick and Ruby, 1997), (ii) Chromosomal gene replacement and marker exchange (Visick and Ruby, 1996), (iii) Transposon mutagenesis using Tn10 (Visick and Ruby, 1997), and two mini-Tn 5 , carrying either a chloramphenicol (Graf and Ruby, 1994) or a streptomycin/spectinomycin (Stabb and Ruby, 1998) resistance cassette, (iv) Antibiotic resistance markers for erythromycin, tetracycline, trimethoprim (from Tn7) and streptomycin/ spectinomycin (Visick and Ruby, 1997; Stabb and Ruby, 1998), (v) Stablely maintained and suicide plasmids (Visick and Ruby, 1996), transferable by triparental mating (Stabb and Ruby, 1998), The Squid-Vibrio Symbiosis as a Biomedical Model 15 (vi) Promoter reporter constructs driving either lacZ or luxAB genes(Visick and Ruby, 1997; 1998a), (vii) Libraries of V. fischeri DNA restricted with EcoRI (Visick and Ruby, 1998b), BglII or XbaI (Stabb and Ruby, 1998), and (viii)A genetic database of over 60 V. fischeri genes and ORFs that has given rise to a reliable codon-usage table (E.G. Ruby, unpublished). Recently developed molecular genetic tools have been used to demonstrate that a number of determinants, including motility (Graf et al., 1994), iron acquisition (Graf and Ruby, 1994), amino acid prototrophy (Graf and Ruby, 1998), luminescence (Visick and Ruby, 1996), and a periplasmic catalase (Visick and Ruby, 1998b), are required by V. fischeri for normal symbiotic competence (Table 1). Similarly, valuable new methods have been devised for the study of the interaction of bacteria with specific E. scolopes tissues and cell types, including: (i) Development of a procedure by which to collect the contents of the light organ crypts (Graf and Ruby, 1996; Nyholm and McFall-Ngai, 1998), (ii) Description of bacteria-induced changes in host cell morphology (Foster and McFall-Ngai, 1998; McFallNgai, 1998b), (iii) Identification of molecular markers for studies of population genetics (Nishiguchi et al., 1998), and (iv) Construction of green-fluorescent-protein (GFP) expression vectors to label and visualize (by confocal laser microscopy) V. fischeri and other Vibrio species in the living host (McFall-Ngai, 1998b). Taken together, these tools and approaches have put the investigation of the squid-vibrio association in a position to make significant advances at levels ranging from population biology and ecology to cell biochemistry and molecular genetics. The Biology of the Association The juvenile squid emerges from its egg free of bacteria (i.e., ‘aposymbiotic’), and must obtain an inoculum of V. fischeri cells to colonize its rudimentary light organ, and thereby become bioluminescent (Wei and Young, 1989; Montgomery and McFall-Ngai, 1994). In nature, inoculation is achieved by the symbiosis-competent V. fischeri cells typically present in the ambient seawater (Lee and Ruby, 1994a; 1995). The beating of cilia that cover two pairs of lateral projections on the surface of the nascent light organ (McFall-Ngai and Ruby, 1991; Montgomery and McFallNgai, 1993) serves to bring bacteria in the seawater close to a series of external pores. These pores lead via ducts to the interior of three pairs of crypts inside the light organ. Within 12 hours a few V. fischeri cells have entered the crypts, and have proliferated to a population size of between 105 and 106 cells (Ruby and Asato, 1993). As it grows and matures, the squid nourishes and maintains within the light organ a V. fischeri population that eventually reaches as many as 10 9 cells. This nonpathogenic infection is characterized by two remarkable traits: the symbiont population remains monospecific, resisting any apparent contamination from other bacteria in the external environment (Montgomery and McFall-Ngai, 1993); and, there is no opportunistic invasion of other host Table 1. Examples of Colonization Determinants that Characterize Both Pathogenic and Benign Infections Pathogenic Vibrio speciesa Proposed Role Colonization regulation Secreted ADPr enzyme Mucus penetration Prototrophic growth Iron acquisition Adhesion Catalase Luminescence Mannose-sensitive adhesion Protease hap regulation a Determinant ToxR c CtxAB c motility f HemA h IrgA j OmpU l —— LuxAB o MshA q Hap s HapR t Colonization phenotypeb + + + + + ? ? ? ? Non-pathogenic V. fischeri Determinant ToxR d HvnA/B e motility g LysA i GlnD k OmpV m KatA n LuxAB p (MshA) r (Hap) HapR u Colonization phenotype ? ? + + + + + + + ? ? This list is a compendium of characteristics attributed to V. cholerae, V. vulnificus, and/or V. parahaemolyticus. The absence of the determinant either is well correlated to a colonization defect (+), is not correlated to a defect (-), or is as yet of an unclear or controversial importance (?). c Salyers and Whitt, 1994 d Reich and Schoolnik, 1994 e Reich and Schoolnik, 1996; Reich et al., 1997 f Richardson, 1991 g Graf et al., 1994 h Rijpkema et al., 1992 i Graf and Ruby, 1998 j Tashima et al., 1996 k Graf and Ruby, 1994; 1996 l Sperandio et al., 1995 m Aeckersberg and Ruby, 1998 n Visick and Ruby, 1998b o Palmer and Colwell, 1991 p Visick and Ruby, 1996; Visick et al., 1996 q Jouravleva et al., 1998 r McFall-Ngai et al., 1998; Feliciano and Ruby, 1999 s Hase and Finklestein, 1993; Wu et al., 1996 t Jobling and Holmes, 1997 u P.M. Fidopiastis and E.G. Ruby, unpublished b 16 Ruby tissues by these V. fischeri cells under normal circumstances (Ruby and McFall-Ngai, 1992; Ruby, 1996). These findings indicate the presence of a specific, tightly regulated recognition between the bacteria and the tissues of their host. In addition, population genetics studies (Lee and Ruby, 1994b) have shown that, while strains of V. fischeri isolated from different Euprymna species are capable of initiating and sustaining a symbiosis with E. scolopes, in mixed inoculations they are outcompeted by native symbiont strains (Nishiguchi et al., 1998). Thus, the squid-vibrio association is both a complex and an ancient one, resulting from a coordinated evolution between the two partners. Ultrastructural studies of both juvenile and adult light organs (McFall-Ngai and Montgomery, 1990; Montgomery and McFall-Ngai, 1993; Nyholm and McFall-Ngai, 1998) have revealed that V. fischeri associates with three types of host cells within the crypts - the highly polarized epithelium and specialized goblet cells that line the crypt space, and a population of amoeboid granulocytes (phagocytes) that moves freely within this space (Figure 2). These cell types all appear to participate in the dynamics of the colonization process. The V. fischeri cells that initiate the colonization must make their way through the ducts leading to the light organ interior, adhere to the epithelium of the crypts, and grow to fill the crypt spaces, utilizing host-secreted organic material. Every day at dawn, the squid expels most of the crypt contents, including 90 to 95% of the bacterial population, through the pores and into the external environment (Ruby and Asato, 1993; Lee and Ruby, 1994a); the remaining 5 to 10% of the bacteria continue to be associated with the microvillous epithelium. During the course of the day, these residual V. fischeri cells proliferate, repopulating the crypts and restoring a full bioluminescence potential for the host’s nocturnal behavior (Boettcher et al., 1996). This continuous cycle of purging and regrowth selects bacterial strains that not only are capable of continued growth within the crypts, but also are competitively dominant under the particular conditions created there (Lee and Ruby, 1994b; Visick and Ruby, 1998b). Because the pores leading to the crypts remain open to the environment throughout the life of the squid, inappropriate species of bacteria that are present in the ambient seawater will continue to have access to the crypts (Lee and Ruby, 1994a). One line of defense against colonization by bacteria other than symbiosis-competent V. fischeri may be the phagocytic cells that are present in the crypts (Nyholm and McFall-Ngai, 1998). The function of these phagocytes is not well defined in the squid-vibrio system, but they are members of the class of cells that are the principal mediators of the immune response in squids (Budelmann et al., 1997). These cells are thus candidates for a role in maintaining the light organ species-specific for V. fischeri, and/or in controlling the size of the symbiont population. Cellular Events Occurring During Colonization Detailed morphological, biochemical and genetic studies have revealed a number of dramatic events that accompany the initiation and persistence of the benign infection of the light organ crypts by V. fischeri cells. During the first few hours to days following the colonization of the crypt epithelium, both partners undergo significant symbiosis-related changes. For instance, the bacteria differentiate into a non-flagellated form (Ruby and Asato, 1993), and their level of luminescence per cell is induced over 1000-fold (Boettcher and Ruby, 1990) due to the accumulation in the crypts of at least one V. fischeri-specific quorum-sensing molecule, an acyl-homoserine lactone called VAI-1 (Fuqua et al., 1996; Boettcher and Ruby, 1995). The squid host undergoes an even more striking developmental program. Morphologically, before colonization is initiated, the nascent light organ has two pairs of ciliated projections on its surface (Montgomery and McFall-Ngai, 1993), and the polarized epithelial cells that line the crypts are columnar and have sparse microvillar projections (Lamarcq and McFall-Ngai, 1998). After V. fischeri cells have initiated their cooperative interaction, the gross morphology of these light organ structures begins to change. Within 8 hours following inoculation the ciliated projections begin to regress through a process of b b v b A B C Figure 2. Transmission Electron Micrographic Images of Host Cell Types Encountered in the Light Organ by Symbiotic Vibrio fischeri (A) Epithelial cells lining the light organ crypts bear dense microvilli in which V. fischeri cells (b) are embedded (bar = 2 µm). (B) Scattered throughout the epithelia, and near the bacteria, are goblet cells that contain a high density of internal vesicles (v), possibly containing glycoproteins (bar = 5 µm). (C) Phagocytic mollusk granulocytes are found within the crypts, moving among the bacterial population (bar = 1 µm). The Squid-Vibrio Symbiosis as a Biomedical Model 17 programmed cell death (Montgomery and McFall-Ngai, 1994; Foster and McFall-Ngai, 1998), and after a few days they have completely disappeared (Doino and McFall-Ngai, 1995). In addition, the epithelial cells lining the crypts have increased 4-fold in volume and become more cuboidal, and their microvilli have increased 4-fold in density and more completely surround and support the bacterial cells (Figure 2A) (McFall-Ngai, 1994; Lamarcq and McFall-Ngai, 1998). There are also significant biochemical changes during this period. Initially, a high concentration of a species of host mRNA encoding haloperoxidase (HPO) (Weis et al., 1996) is present in the uncolonized light organ. However, once the organ is colonized, the level of HPO drops significantly (Small and McFall-Ngai, 1998), thus reducing the potential for the production of the antibacterial compound hypohalous acid (Forman and Thomas, 1986; Tomarev et al ., 1993; Boettcher, 1994). The host developmental program is not initiated in the absence of a symbiotic infection (McFall-Ngai and Ruby, 1991); however, once triggered, some (but not all) of the events will proceed without the continued presence of the bacteria (Doino and McFall-Ngai, 1995; Lamarcq and McFall-Ngai, 1998). As a result, a distinct, functional organ is formed through a program of biochemical accommodation and cooperation that is analogous to the well described development of the Rhizobium -legume symbiosis (van Rhijn and Vanderleyden, 1995). Obstacles to Bacterial Colonization To colonize an animal host, bacteria, whether pathogenic or cooperative, must overcome characteristics of the tissue that discourage the growth of inappropriate microorganisms. These obstacles to colonization may include general environmental conditions in the tissue, as well as active responses by the host’s defenses. Five such challenges that appear to play a role in colonization of the light organ crypts are summarized below. Viscous Barrier Both flagellated and unflagellated motility mutants of V. fischeri are unable to initiate a colonization, even when presented as a 1000-fold higher inoculum, and for a 10times longer exposure (Graf et al., 1994). The cilia lining the duct leading to the crypts create an outward-directed current (McFall-Ngai and Ruby, 1998), and the presence of mucopolysaccharides in the ducts (Nyholm and McFallNgai, 1998) and emanating from the pore (Figure 3) suggests that the cells must penetrate a viscous barrier to reach the crypts themselves. m Figure 3. Mucus-like Material Around the Pore Leading to the Light Organ Crypts of Euprymna scolopes Transmission electron micrographs reveal a flocculant, glycoprotein-like material (m) surrounding the ciliated cells that form the pore (arrow) leading into the crypt spaces (bar = 1 µm). 18 Ruby Nutritional Limitation Proteins and/or peptides in the crypt contents may constitute a substantial source of nutrients: the ability of some amino acid auxotrophs to fully colonize the crypts indicates that the host must be a major source of these building blocks (Graf and Ruby, 1998). While it is not yet known whether there is a specific bacterial signal that is needed to elicit the host’s production and export of these nutrients, two morphological observations of the colonized light organ are suggestive. First, the presence of goblet cells has been noted in both the duct and crypt regions of the light organ (Figure 2B). These goblet cells could be the source of mucopolysaccharide glycoproteins, like mucin, that might be secreted into the crypts. Second, the epithelial cells lining the crypt walls become edematous, swelling to about 4-fold the volume of the cells lining uninfected crypts (Montgomery and McFall-Ngai, 1994). Such swelling is indicative of osmotic stress in epithelial cells, which may lead to the leaking of organic materials into the surrounding extracellular spaces. Clearing by Expulsion The diurnal process of expulsion will select for symbionts that are able to attach to the epithelial cells lining the crypt spaces. Evidence exists to suggest that a mannosesensitive adhesion is important for successful colonization (McFall-Ngai et al., 1998; Feliciano and Ruby, 1999). In addition, the increasing intimacy observed between V. fischeri cells and the epithelial crypt microvilli as the association matures (Lamarcq and McFall-Ngai, 1998) suggests that bacterial outer membrane proteins (Aeckersberg and Ruby, 1998) or other surface components become increasingly important for persistent attachment. Exposure to Reactive Oxygen Species Recent data indicate that the uninfected crypt environment is oxidatively stressful; for instance, elevated superoxide anion production has been detected in the light organ tissue (Small and McFall-Ngai, 1993). In addition, the squid light organ has unusually high levels of mRNA encoding a HPO with significant sequence similarity to human myeloperoxidase, an antimicrobial protein in neutrophils (Tomarev et al., 1993) that catalyzes the conversion of H2O2 and halide ions into microbicidal hypohalous acids. Activity assays and immunocytochemical analyses have demonstrated that HPO is present in the crypt space, and occurs at elevated levels elsewhere in the squid where there are pathogenic bacterial infections (Small and McFallNgai, 1998). Removal by Phagocytes One line of defense against colonization that V. fischeri cells must avoid appears to be the phagocytic cells in the crypts (Figure 2C) (Nyholm and McFall-Ngai, 1998). Such cells arise in a tissue called the white body and are typically found in the hemolymph (Budelmann et al., 1997) and in extracellular spaces like the light organ crypts. Phagocytes of invertebrates share several ultrastructural, physiological and biochemical traits with mammalian cells such as neutrophils and macrophages that are associated with innate immunity (Sminia et al., 1987; Greger et al., 1996). These traits underlie the common responses of these cells to microorganisms, such as respiratory burst activity and phagocytosis. Such common response pathways, which can function through the release of chemokines and cytokines (e.g., interleukins and tumor necrosis factoralpha), are indicative of an ancient origin for the mechanisms by which animal cells and microorganisms interact (Belvin and Anderson, 1996; May and Ghosh, 1998). Continuing studies of these and other host-imposed barriers are an important focus of research into the basis of the reciprocal accommodation reached between V. fischeri and its host during the development of the light organ association. Similarities between Benign and Pathogenic Tissue Colonization In recent years it has become clearer that certain virulence determinants of pathogenic Vibrio species are expressed in, and may be required for, the benign V. fischeri colonization. The list of similarities between the colonization of host epithelium by V. fischeri and that of a number of pathogenic Vibrio species such as V. vulnificus , V. parahaemolyticus and V. cholerae continues to grow (Table 1). These similarities include known virulence determinants of V. cholerae (ToxR and CtxAB) that have protein homologs or analogs in V. fischeri (Reich and Schoolnik, 1994; Reich and Schoolnik, 1996; Reich et al., 1997). Because of these similarities, and the phylogenetic relatedness of V. fischeri and pathogenic Vibrio species, it is reasonable to hypothesize that a number of properties of their associations with host tissue might have arisen from a common evolutionary origin. In fact, some species, like V. vulnificus and V. harveyi, can be a benign symbiont in one host and a virulent pathogen in another (DePaola et al., 1994; Harris et al., 1996) suggesting a duality of function in these different classes of association. This hypothesis is further supported by additional evidence of the homologous character of tissue colonization by V. fischeri and pathogenic Vibrio species. The identity of these V. fischeri genetic and enzymatic homologs suggests that they are important in three critical and interacting aspects of epithelial colonization: bacterial attachment, bacterial avoidance of host defense responses, and host provision of nutrition to the bacterial culture (Table 1). Because the commensal colonization of invertebrate tissues by Vibrio species arose millions of years before the development of human pathogenesis by members of this genus it seems likely that host-colonization mechanisms that were present in these cooperative associations may have been recruited into the development of pathogenic associations. A similar argument has been made to explain the existence of a majority of the known Salmonella virulence determinants in nonpathogenic Escherichia coli, the organism in which many of these determinants were first discovered and described (Groisman and Ochman, 1994) The epithelial cell surfaces of the human body harbor a complex and essential community of bacterial species. These bacteria not only create a normal, healthy biochemical microenvironment on these surfaces, but also provide the first line of defense against invading pathogens. The growing awareness of the essential role that the natural microbiota play in the development and maintenance of the healthy state in animals (Hooper et al., 1998) has led to an increasing recognition of the importance of benign bacterial colonization of tissues. To design effective The Squid-Vibrio Symbiosis as a Biomedical Model 19 therapeutic measures against potential diseases, we must understand the dynamic interactions between hosts and their essential microbial partners. However, little is known either about the signaling processes, and their genetic regulation, through which these beneficial bacteria establish stable associations with host cells, or about how these very same mechanisms are exploited by harmful bacteria. The light organ symbiosis between E. scolopes and V. fischeri offers unique opportunities to study these processes. Important characteristics of this natural experimental model that make it particularly useful for comparisons of benign and pathogenic colonization of animal tissue are: (i) the laboratory culturability of both partners (Wei and Young, 1989; Boettcher and Ruby, 1990; Hanlon et al., 1997); (ii) the specificity of the association (McFall-Ngai and Ruby, 1991); (iii) the similarity between the microvillous, polarized epithelial cells and phagocytes of the light organ crypts and those of the human intestinal lining (McFall-Ngai and Montgomery, 1990; Nyholm and McFall-Ngai, 1998); and, (iv) the close genetic relationship between V. fischeri and pathogenic relatives, such as V. vulnificus and V. cholerae. As in most associations between bacteria and their hosts, this benign infection does not produce a diseased state in the colonized tissue; thus, the signals and responses of the two partners follow a predictable and integrated pattern. However, unlike the most common types of associations (e.g., the normal microbiota of the enteric tract, skin and oral cavity), the V. fischeri light organ colonization is monospecific, thereby greatly simplifying the elucidation of bacteria-host interactions. To date, success with this model has resulted from collaborations involving bacterial molecular physiologists and geneticists, and animal developmental biochemists. By continuing to integrate efforts to understand the responses of each member of this association to the other, the chances of making important discoveries about bacterial tissue colonization will be enhanced. Future Directions Further investigations of the symbiotic colonization of E. scolopes by V. fischeri will promote a paradigm for studying host-bacterial interactions that will specifically serve to advance biomedical objectives in the following ways. (i) Defining the biochemical and molecular events that can characterize the specific initiation, rapid colonization, and long-term persistence of benign bacterial infections is of increasing importance. While a number of other promising model systems are emerging (Forst and Nealson, 1996; Whittaker et al., 1996; Hooper et al., 1998; Graf, 1999), the squid-vibrio association is already becoming well-established (Ruby, 1996; McFall-Ngai, 1998b). (ii) The importance of the kind of bacterial signaling called “quorum-sensing” (Fuqua et al., 1996) in pathogenic bacterial-host signal interactions has become increasing evident. V. fischeri, the species in which this mechanism was first discovered, continues to be the paradigm system for the study of both intra- and interspecies association (Visick and Ruby, 1999). (iii) Continued studies of bacterial and host determinants that potentiate light organ symbiosis may not only reveal further convergences with known pathogenic virulence factors (Table 1) but, more importantly, may also promote the discovery of as yet undescribed ones (Fidopiastis and Ruby, 1999). In addition, the level of understanding of the ecology of V. fischeri (Ruby and Lee, 1998) makes this species particularly wellpositioned for use in testing experimental models of the evolution of horizontally transmitted pathogens (Ebert, 1998). (iv) Future discoveries of the biology of the squid-vibrio association may aid in efforts to understand the mechanisms by which benign colonizations of mollusk tissue serve as a reservoir for human pathogenic Vibrio species (Tamplin and Capers, 1992), and to determine the role of such reservoirs in the recent resurgence of pathogenic epidemics (Colwell, 1996). Acknowledgements I thank M. McFall-Ngai and her laboratory for their critical collaborative interactions, and specifically for all the images used in this review. I also thank the members of my laboratory for producing much of the data I’ve discussed. Portions of the research described herein were funded by the National Institutes of Health through grant RO1-RR12294 to E.G.R. and M. McFallNgai, and by National Science Foundation grant IBN96-01155 to M. McFallNgai and E.G.R. References Aeckersberg, F., and Ruby, E.G. 1998. Possible role of an outer membrane protein of Vibrio fischeri in its symbiotic infection of Euprymna scolopes. Abstr. Gen. Meet. Amer. Soc. Microbiol. 98: 374. Belvin, M. P., and Anderson, K.V. 1996. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Ann. Rev. Cell Dev. Biol. 12: 393-416. Berry, S.S. 1912. The Cephalopoda of the Hawaiian Islands. Bull. U.S. Bur. Fish. 32: 255-362. Blake, P.A. 1983. Vibrios on the half shell: what the walrus and the carpenter didn’t know. Ann. Intern. Med. 99: 558-559. Boettcher, K.J. 1994. Regulatory consequences of a high density bacterial population in the Euprymna scolopes-Vibrio fischeri light organ symbiosis. Doctoral dissertation, Univ. So. Calif. p. 1551. Boettcher, K.J., and Ruby, E.G. 1990. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid, Euprymna scolopes. J. Bacteriol. 172: 3701-3706. Boettcher, K.J., and Ruby, E.G. 1995. Detection and quantification of Vibrio fischeri autoinducer from symbiotic squid light organs. J. Bacteriol. 177: 1053-1058. Boettcher, K.J., Ruby, E.G., and McFall-Ngai, M.J. 1996. Bioluminescence in the symbiotic squid Euprymna scolopes is controlled by a daily biological rhythmn. J. Comp. Physiol. 179: 65-73. Budelmann, B.U, Schipp, R., and Boletzky, S. v. 1997. Cephalopoda, Chp. 3. In: Microscopic Anatomy of the Invertebrates, Vol. 6A: Mollusca II. F. W. Harrison and A. J. Kohn, eds. Wiley, Liss, Inc., N.Y. p. 119-414. Breznak, J.A., and Brune, A. 1994. Role of microorganisms in the digestion of lignocellulose by termites. Annu. Rev. Entomol. 39: 453-487. Colwell, R.R. 1996 Global climate and infectious disease: the cholera paradigm. Science. 274: 2025-2031. DePaola, A., Capers, G.M., and Alexander, D. 1994. Densities of Vibrio vulnificus in the intestines of fish from the U.S. Gulf Coast. Appl. Environ. Microbiol. 60: 984-988. Doino, J.A., and McFall-Ngai, M.J. 1995. Transient exposure to competent bacteria initiates symbiosis-specific squid light organ morphogenesis. Biol. Bull. 189: 347-355. Dougan, G. 1994. The molecular basis for the virulence of bacterial pathogens: implications for oral vaccine development. Microbiol. 140: 215224. Ebert, D. 1998. Experimental evolution of parasites. Science. 282: 14321435. Feliciano, B., and Ruby, E.G. 1999. Identification and characterization of the Vibrio fischeri hemagglutination factor. Abstr. Gen. Meet. Amer. Soc. Microbiol. 99: 462. Fidopiastis, P.M., Boletzky, S.v., and Ruby, E.G. 1998. A new niche for Vibrio logei, the predominant light organ symbiont of squids in the genus Sepiola. J. Bacteriol. 180: 59-64. Fidopiastis, P.M., and Ruby, E.G. 1999. Cryptic luminescence in the cold- 20 Ruby water fish pathogen Vibrio salmonicida. Arch. Microbiol. 171: 205-209. Forman, H.J., and Thomas, M.J. 1986. Oxidant production and bacteriocidal activity of phagocytes. Ann. Rev. Physiol. 48: 669-680. Forst, S., and Nealson, K.H. 1996. Molecular biology of the symbioticpathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiol. Rev. 60: 21-43. Foster, J. S., and McFall-Ngai, M.J. 1998. Induction of apoptosis by cooperative bacteria in the morphogenesis of host epithelial tissues. Dev. Genes Evol. 208: 295-303. Fuqua, W.C., Winans, S.C., and Greenberg, E.P. 1996. Census and concensus in bacterial ecosystems: the LuxR-LuxI family of quorumsensing transcriptional regulators. Annu. Rev. Microbiol. 50: 727-751. Graf, J. 1999. Symbiosis of Aeromonas veronii biovar sobria and Hirudo medicinalis, the medicinal leech: a novel model for digestive tract associations. Infect. Immun. 67: 1-7. Graf, J., Dunlap, P.V., and Ruby, E.G. 1994. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J. Bacteriol. 176: 6986-6991. Graf, J., and Ruby, E.G. 1994. The effect of iron-sequestration mutations on the colonization of Euprymna scolopes by symbiotic Vibrio fischeri. Abstr. Ann. Meet. Amer. Soc. Microbiol. 94: 76. Graf, J., and Ruby, E.G. 1996. Characterization of a Vibrio fischeri glnD mutant: both iron and nitrogen utilization are affected. Abstr. Ann. Meet. Amer. Soc. Microbiol. 96: 509. Graf, J., and Ruby, E.G. 1998. Host-derived amino acids support the proliferation of symbiotic bacteria. Proc. Natl. Acad. Sci. USA. 95: 18181822. Greger, E., Drum, A., and Elston, R. 1996. Lucigenin- and luminol-dependent chemiluminescent measurement of oxyradical production in hemocytes of the Pacific razor clam, Siliqua patula, and the oyster, Crassostrea gigas, Chp. 16. In: Modulators of Immune Responses: The Evolutionary Trail. J.S. Stolen, T.C. Fletcher, C.J. Bayne, C.J. Secombes, J.T. Zelikoff, L.E. Twerkok, and D.P. Anderson, eds. SOS Publ., Fair Haven, N.J. p. 203208. Groisman, E.A., and Ochman, H. 1994. How to become a pathogen. Trends Microbiol. 2: 289-294. Hanlon, R.T., Claes, M.F., Ashcraft, S.E., and Dunlap, P.V. 1997. Laboratory culture of the sepiolid squid Euprymna scolopes: a model system for bacteria-animal symbiosis. Biol. Bull. 192:364-374. Harris, L., Owens, L., and Smith, S. 1996. A selective medium for Vibrio harveyi. Appl. Environ. Microbiol. 62: 3548-3550. Hase, C.C., and Finkelstein, R.A. 1993. Bacterial extracellular zinccontaining metalloproteases. Microbiol. Rev. 57: 823-837. Hooper, L.V., Bry, L., Falk, P.G., and Gordon, J.I. 1998. Host-microbial symbiosis in the mammalian intestine: exploring an internal ecosystem. BioEssays. 20: 336-343. Jobling, M.G., and Holmes, R.K. 1997. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol. Microbiol. 26: 1023-1034. Jouravleva, E.A., McDonald, G.A., Marsh, J.W., Taylor, R.K., BoesmanFinkelstein, M., and Finkelstein, R.A. 1998. The Vibrio cholerae mannosesensitive hemagglutinin is the receptor for a filamentous bacteriophage from V. cholerae O139. Infect. Immun. 66: 2535-2539. Lamarcq, L.H., and McFall-Ngai, M.J. 1998. Induction of a gradual, reversible morphogenesis of its host’s epithelial brush border by Vibrio fischeri. Infect. Immun. 66: 777-785. Lee, K.-H., and Ruby, E.G. 1994a. Effect of the squid host on the abundance and distribution of symbiotic Vibrio fischeri in nature. Appl. Environ. Microbiol. 60: 1565-1571. Lee, K.-H., and Ruby, E.G. 1994b. Competition between Vibrio fischeri strains during the initiation and maintenance of a light organ symbiosis. J. Bacteriol. 176: 1985-1991. Lee, K.-H., and Ruby, E.G. 1995. Symbiotic role of the nonculturable, but viable, state of Vibrio fischeri in Hawaiian seawater. Appl. Environ. Microbiol. 61: 278-283. May, M.J., and Ghosh, S. 1998. Signal transduction through NF-kappaB. Immunol. Today. 19: 80-88. McFall-Ngai, M.J. 1990. Crypsis in the pelagic environment. Amer. Zool. 30: 175-188. McFall-Ngai, M.J. 1994. Evolutionary morphology of a squid symbiosis. Amer. Zool. 34: 554-561. McFall-Ngai, M.J. 1998a. The development of cooperative associations between animals and bacteria: establishing detente among Domains. Amer. Zool. 38: 593-608. McFall-Ngai, M.J. 1998b. Pioneering the squid-vibrio model. ASM News. 64: 639-645. McFall-Ngai, M.J., and Montgomery, M. 1990. The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda:Sepiolidae). Biol. Bull. 179: 332-339. McFall-Ngai, M.J., and Ruby, E.G. 1991. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial symbiosis. Science. 254: 1491-1494. McFall-Ngai, M.J., and Ruby, E.G. 1998. Bobtail squid and their luminous bacteria: when first they meet. BioScience. 48: 257-265. McFall-Ngai, M.J., Brennan, C., Weis, V., and Lamarcq, L. 1998. Mannose adhesin-glycan interactions in the Euprymna scolopes-Vibrio fischeri symbiosis. In: New Developments in Marine Biotechnology. Y. Le Gal and H. Halvorson, eds. Plenum Press, N.Y. p. 273-276. Meighen, E.A. 1991. Molecular biology of bacterial bioluminescence. Microbiol. Rev. 55: 123-142. Montgomery, M.K., and McFall-Ngai, M.J. 1992. The muscle-derived lens of a squid bioluminescent organ is biochemically convergent with the ocular lens. Evidence for recruitment of ALDH as a predominant structural protein. J. Biol. Chem. 267: 20999-21003. Montgomery, M.K., and McFall-Ngai, M.J. 1993. Embryonic development of the light organ of the sepiolid squid Euprymna scolopes. Biol. Bull. 184: 296-308. Montgomery, M.K., and McFall-Ngai, M.J. 1994. The effect of bacterial symbionts on early post-embryonic developmental of a squid light organ. Development. 120: 1719-1729. Montgomery, M.K., and McFall-Ngai, M.J. 1998. Late postembryonic development of the symbiotic light organ of Euprymna scolopes (Cephalopoda:Sepiolidae). Biol. Bull. 195: 326-336. Nealson, K.H., and Hastings J.W. 1991. The luminous bacteria. In: The Prokaryotes, a Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications. 2nd ed. A. Balows, H.G. Truper, M. Dworkin, W. Harder, and K.H. Schleifer, eds. Springer-Verlag, N.Y. p. 625639. Nesis, K.N. 1987. Cephalopods of the World. T.F.H. Publications, Neptune City, N.J. Nishiguchi, M., Ruby, E.G., and McFall-Ngai, M.J. 1998. Competitive dominance among strains of luminous bacteria provides an unusual form of evidence for parallel evolutionin sepiolid squid-vibrio symbioses. Appl. Environ. Microbiol. 64: 3209-3213. Nyholm, S.V., and McFall-Ngai, M.J. 1998. Sampling the light organ microenvironment of Euprymna scolopes: Description of a population of host cells in association with the bacterial symbiont. Biol. Bull. 195: 89-97 Palmer, L.M., and Colwell, R.R. 1991. Detection of luciferase gene sequence in nonluminescent Vibrio cholerae by colony hybridization and polymerase chain reaction. Appl. Environ. Microbiol. 57: 1286-1293. Reich, K.A., and Schoolnik, G.K. 1994. The light organ symbiont Vibrio fischeri possesses a homolog of the Vibrio cholerae transmembrane transcriptional activator ToxR. J. Bacteriol. 176: 3085-3088. Reich, K.A., and Schoolnik, G.K. 1996. Halovibrin, secreted from the light organ symbiont Vibrio fischeri, is a member of a new class of ADPribosyltransferases. J. Bacteriol. 178: 209-215. Reich, K.A., Beigel, T., and Schoolnik, G.K. 1997. The light organ symbiont Vibrio fischeri possesses two distinct secreted ADP-ribosyltransferases. J. Bacteriol. 179: 1591-1597. Richardson, K. 1991. Roles of motility and flagellar structure in pathogenicity of Vibrio cholerae: analysis of motility mutants in three animal models. Infect. Immun. 59: 2727-2736. Rijpkema, S.G.T., Bik, E.M., Jensen, W.H., Gielen, H., Versluis, L.F., Stouthamer, A.H., Guinee, P.A.M., and Mooi, F.R. 1992. Construction and analysis of a Vibrio cholerae δ-aminolevulinic acid auxotroph which confers protective immunity in a rabbit model. Infect. Immun. 60: 2188-2193. Rook, G.A., and Stanford, J.L. 1998. Give us this day our daily germs. Immunol. Today. 19: 113-116. Ruby, E.G. 1996. Lessons from a cooperative bacterial-animal association: The Vibrio fischeri-Euprymna scolopes light organ symbiosis. Annu. Rev. Microbiol. 50: 591-624. Ruby, E.G. 1999. Ecology of a benign “infection”: colonization of the squid luminous organ by Vibrio fischeri. In: Microbial Ecology and Infectious Disease. E. Rosenberg, ed. Amer. Soc. Microbiol. Press, Washington. p. 217-231. Ruby, E.G., and Asato, L.M. 1993. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch. Microbiol. 159: 160-167. Ruby, E.G., and Lee, K.-H. 1998. The Vibrio fischeri -Euprymna scolopes light organ association: current ecological paradigms. Appl. Environ. Microbiol. 64: 805-812. Ruby, E.G., and McFall-Ngai, M.J. 1992. A squid that glows in the night: development of an animal-bacterial mutualism. J. Bacteriol. 174: 48654870. Salyers, A.A., and Whitt, D.D. 1994. Bacterial Pathogenesis: a Molecular Approach. Amer. Soc. Microbiol. Press, Washington, D.C. Small, A.L., and McFall-Ngai, M.J. 1993. Changes in the oxygen The Squid-Vibrio Symbiosis as a Biomedical Model 21 environment of a symbiotic squid light organ in response to infection by its luminous bacterial symbionts. Amer. Zool. 33: 61A Small, A.L., and McFall-Ngai, M.J. 1998. A halide peroxidase in tissues that interact with bacteria in the host squid Euprymna scolopes. J. Cellular Biochem. 72: 445-457. Sminia, T., and van der Knaap, W.P.W. 1987. Cells and molecules in molluscan immunology. Dev. Comp. Immunol. 11: 17-28. Sperandio, V., Giron, J.A., Silveira, W.D., and Kaper, J.B. 1995. The OmpU outer membrane protein, a potential adherence factor of Vibrio cholerae. Infect. Immun. 63: 4433-4438. Stabb, E.V., and Ruby, E.G. 1998. Construction of a mobilizable Tn5 derivative and its utility for mutagenizing, cloning and mapping loci in the luminescent symbiont Vibrio fischeri. Abstr. Ann Meet. Amer. Soc. Microbiol. 98: 375. Tamplin, M.L., and Capers, G.M. 1992. Persistence of Vibrio vulnificus in tissues of Gulf Coast oysters, Crassostrea virginica, exposed to seawater disinfected with UV light. Appl. Environ. Microbiol. 58: 1506-1510. Tashima, K.T., Carroll, P.A., Rogers, M.B., and Calderwood, S.B. 1996. Relative importance of three iron-regulated outer membrane proteins for in vivo growth of Vibrio cholerae. Infect. Immun. 64: 1756-1761. Tomarev, S.I., Zinovieva, R.D., Weis, V.M., Chepelinsky, A.B., Piatigorsky, J., and McFall-Ngai, M.J. 1993. Abundant mRNAs in the bacterial light organ of a squid encode a protein with high similarity to mammalian antimicrobial peroxidases: Implications for mutualistic symbioses. Gene. 132: 219-226. van Rhijn, P., and Vanderleyden, J. 1995. The Rhizobium-plant symbiosis. Microbiol. Rev. 59: 124-142. Visick, K.L., Orlando, V.I., and Ruby, E.G. 1996. Role of the luxR and luxI genes in the Vibrio fischeri-Euprymna scolopes symbiosis. Abstr. Ann. Meet. Amer. Soc. Microbiol. 96: 509. Visick, K.L., and Ruby, E.G. 1996. Construction and symbiotic competence of a luxA-deletion mutant of Vibrio fischeri. Gene. 175: 89-94. Visick, K.L., and Ruby, E.G. 1997. New genetic tools for use in the marine bioluminescent bacterium Vibrio fischeri. In: Bioluminescence and Chemiluminescence, Proceedings of the 9th International Symposium 1996. J.W. Hastings, L.J. Kricka and P.E. Stanley, eds. Wiley and Sons, N.Y. p. 119-122. Visick, K.L., and Ruby, E.G. 1998a. Temporal control of lux gene expression in the symbiosis between Vibrio fischeri and its squid host. In: New Developments in Marine Biotechnology. Y. Le Gal and H. O. Halvorson, eds. Plenum Press, N.Y. p. 277-279. Visick, K.L., and Ruby, E.G. 1998b. The periplasmic, group III catalase of Vibrio fischeri is required for normal symbiotic competence and is induced both by oxidative stress and approach to stationary phase. J. Bacteriol. 180: 2087-2092. Visick, K.L., and Ruby, E.G. 1999. The emergent properties of quorum sensing: consequences to bacteria of autoinducer signaling in their natural environment. In: Cell-Cell Signaling in Bacteria. G.M. Dunny and S.C. Winans, eds. Amer. Soc. Microbiol. Press, Washington, D.C. p. 333-352. Wei, S.L., and Young, R.E. 1989. Development of symbiotic bacterial luminescence in a nearshore cephalopod, Euprymna scolopes. Mar. Biol. 103: 541-546. Weis, V.M., Small, A.L., and McFall-Ngai, M.J. 1996. A peroxidase related to the mammalian antimicrobial protein myeloperoxidase in the EuprymnaVibrio mutualism. Proc. Natl. Acad. Sci. USA. 93: 13683-13688. Weis, V., Montgomery, M.K., and McFall-Ngai, M.J. 1993. Enhanced production of ALDH-like protein in the bacterial light organ of the sepiolid squid Euprymna scolopes. Biol. Bull. 184: 309-321. Whittaker, C.J., Klier, C.M., and Kolenbrander, P.E. 1996. Mechanisms of adhesion by oral bacteria. Annu. Rev. Microbiol. 50: 513-552. Wu, Z., Milton, D., Nybom, P., Sjo, A., and Magnusson, K.-E. 1996. Vibrio cholerae hemaglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microbial Path. 21: 111-123. 22 Ruby