* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Synergistic stabilization of BCS Class II drug
Polysubstance dependence wikipedia , lookup
Orphan drug wikipedia , lookup
Compounding wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Nicholas A. Peppas wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Drug design wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
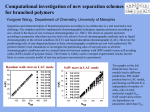
Synergistic stabilization of BCS Class II drug nanosuspensions via combination of cellulosic polymers and anionic surfactants Meng Li, Afolawemi Afolabi, Anthony Quarato, Ecevit Bilgili Department of Chemical, Biological and Pharmaceutical Engineering, New Jersey Institute of Technology Abstract: Ensuring the physical stability of drug nanosuspensions prepared via wet media milling has been a challenge for pharmaceutical scientists. The aim of this study is to assess the combined use of non-ionic cellulosic polymers and anionic surfactants in stabilizing multiple drug nanosuspensions. Particle size of five drugs, i.e. azodicarbonamide (AZD), fenofibrate (FNB), griseofulvin (GF), ibuprofen (IBU) and phenylbutazone (PB) was reduced separately in an aqueous solution of hydroxypropyl cellulose (HPC) with/without sodium dodecyl sulfate (SDS) via a stirred media mill. Laser diffraction, scanning electron microscopy, thermal analysis, rheometry and electrophoresis were used to evaluate the breakage kinetics, storage stability, electrostatic repulsion and stabilizer adsorption. Without SDS, drug particles exhibited aggregation to different extents; FNB and GF particles aggregated the most due to low zeta potential and insufficient steric stabilization. Although aggregation in all milled suspensions was reduced due to HPC–SDS combination, FNB and IBU showed notable growth during 7-day storage. It is concluded that the combination of non-ionic cellulosic polymers and anionic surfactants is generally viable for ensuring the physical stability of wet-milled drug nanosuspensions, provided that the surfactant concentration is optimized to mitigate the Ostwald ripening, whereas cellulosic polymers alone may provide stability for some drug suspensions.