* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Martindale: The Complete Drug Reference
Drug interaction wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug design wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
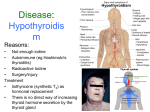
Martindale: The Complete Drug Reference Liothyronine Sodium Date of monograph review: 18-Feb-1997; 01-Jun-1998; 06-Apr-2000; 20Jun-2001; 25-Apr-2002; 19-May-2005; 28-Jun-2006; 13-Aug-2008; 22May-2009; 02-Sep-2010; (latest modification: 14-May-2011) Drug Nomenclature (Latest modification: 20-Apr-2011) Synonyms: 3,5,3′-Tri-iodo-L-thyronine Sodium; Liothyronin sodná sůl; Liothyronine sodique; Liothyroninum Natricum; Liotironin Sodyum; Liotironin-nátrium; Liotironina sódica; Liotironino natrio druska; Liotyroniininatrium; Liotyronina sodowa; Liotyroninnatrium; Sodium Liothyronine; L-Tri-iodothyronine Sodium; Tri-iodotironin Sodyum; リオチロニンナトリウム BAN: Liothyronine Sodium [BANM] INN: Liothyronine Sodium [rINNM (en)] INN: Liotironina sódica [rINNM (es)] INN: Liothyronine Sodique [rINNM (fr)] INN: Natrii Liothyroninum [rINNM (la)] INN: Натрий Лиотиронин [rINNM (ru)] Chemical name: Sodium 4-O-(4-hydroxy-3-iodophenyl)-3,5-di-iodo-Ltyrosine Molecular formula: C15H11I3NNaO4 =673.0 CAS: 6893-02-3 (liothyronine); 55-06-1 (liothyronine sodium); 8065-29-0 (liotrix) ATC code: H03AA02 ATC code (veterinary): QH03AA02 UNII code: GCA9VV7D2N Martindale code: 9006-d Martindale: The Complete Drug Reference Chemical Structure of Liothyronine NOTE: The abbreviation T3 is often used for endogenous tri-iodothyronine in medical and biochemical reports. Liotrix is USAN for a mixture of liothyronine sodium with levothyroxine sodium. Pharmacopoeias: In Eur. (see ), Jpn, and US. Ph. Eur. 7.2 (Liothyronine Sodium). A white or slightly coloured, hygroscopic powder. Practically insoluble in water; slightly soluble in alcohol. It dissolves in dilute solutions of alkali hydroxides. Store at 2 degrees to 8 degrees in airtight containers. Protect from light. USP 34 (Liothyronine Sodium). A light tan, odourless, crystalline powder. Very slightly soluble in water; slightly soluble in alcohol; practically insoluble in most other organic solvents. Store in airtight containers. Adverse Effects, Treatment, and Precautions (Latest modification: 04Mar-2006) As for Levothyroxine Sodium, . Interactions (Latest modification: 04-Mar-2006) As for Levothyroxine Sodium, . Pharmacokinetics (Latest modification: 04-Mar-2006) Martindale: The Complete Drug Reference Liothyronine is readily and almost completely absorbed from the gastrointestinal tract. Once in the circulation, liothyronine binds principally to thyroxine-binding globulin (TBG), although less strongly than levothyroxine; some is also bound to thyroxine-binding pre-albumin (TBPA) or to albumin. Liothyronine has a plasma half-life in euthyroidism of about 1 to 2 days; the half-life is prolonged in hypothyroidism and reduced in hyperthyroidism. Liothyronine is metabolised by deiodination to inactive di-iodothyronine and mono-iodothyronine. Iodine released by deiodination is largely reused within the thyroid cells. Further metabolites result from deamination and decarboxylation to tiratricol (triac). Uses and Administration (Latest modification: 27-Aug-2010) Liothyronine is a thyroid hormone (see hypothyroidism ( ). It is used in the treatment of ), and is believed to be more active than levothyroxine ( ). The onset of action of liothyronine is rapid, developing within a few hours, and therefore it tends to be used in circumstances where this, and its short duration of action, are useful, particularly in hypothyroid (myxoedema) coma. With regular dosing the peak therapeutic effect is usually achieved after 3 days; on withdrawal its effects may persist for 1 to 3 days. The dose of liothyronine should be individualised on the basis of clinical response and biochemical tests and should be monitored regularly. Although liothyronine is given as the sodium salt, doses can be expressed in terms of liothyronine sodium or liothyronine; the doses below are in terms of liothyronine sodium. Liothyronine sodium 10.3 micrograms is equivalent to about 10 micrograms of liothyronine. Liothyronine sodium 20 to 25 micrograms is generally considered to be equivalent in activity to about 100 micrograms of levothyroxine sodium. Martindale: The Complete Drug Reference In hypothyroidism a usual initial oral dose is 5 to 25 micrograms daily, increased gradually to a maintenance dose of 60 to 75 micrograms daily in 2 or 3 divided doses, although up to 100 micrograms daily may be required in some patients. In elderly patients, in those with cardiovascular disorders, or in those with severe long-standing hypothyroidism, treatment should be introduced with doses at the low end of the range, with smaller increments, and longer intervals between increases, as necessary. In hypothyroid coma liothyronine sodium may be given intravenously in a dose of 5 to 20 micrograms by slow intravenous injection, repeated as necessary, usually at intervals of 12 hours; the minimum interval between doses is 4 hours. An alternative regimen advocates an initial dose of 50 micrograms intravenously followed by further injections of 25 micrograms every 8 hours until improvement occurs; the dosage may then be reduced to 25 micrograms intravenously twice daily. For the use of liothyronine in children, see . Liothyronine has also been given in the diagnosis of hyperthyroidism in adults. Failure to suppress the uptake of radio-iodine after several days of receiving liothyronine sodium suggests a diagnosis of hyperthyroidism. For use as augmentation treatment with antidepressants, see Depression under Levothyroxine ( ). Liothyronine hydrochloride has also been used. Administration in children (Latest modification: 31-Aug-2010) In the USA, liothyronine sodium may be given orally for the treatment of congenital hypothyroidism. An initial dose of 5 micrograms daily may be increased by 5 micrograms every 3 to 4 days, according to response, to the following usual doses: Martindale: The Complete Drug Reference children below 1 year may require 20 micrograms daily children between 1 to 3 years may require 50 micrograms daily children over 3 years: as for adults, see In the UK, liothyronine sodium is unlicensed for use in children. However, the BNFC 2010/11 recommends that children aged 12 to 18 years may be given the same dose as adults for the management of hypothyroid coma (see ). Preparations (Latest modification: 19-Apr-2011) Single-ingredient Preparations (Latest modification: 19-Apr-2011) The symbol ¤ denotes a preparation which is discontinued or no longer actively marketed. Australia: Tertroxin; Belgium: Cytomel¤; Brazil: Cynomel¤; Canada: Cytomel; Czech Republic: Tertroxin¤; France: Cynomel; Germany: Thybon; Thyrotardin; Greece: Cynomel; T3; Ireland: Tertroxin; Italy: Dispon¤; Liotir; Ti-Tre; Mexico: Cynomel; Liotrex¤; Triyodisan¤; Triyotex; Netherlands: Cytomel; New Zealand: Tertroxin; Portugal: NeoTiroimade; South Africa: Tertroxin; Thailand: Tertroxin¤; Turkey: Tiromel; United Kingdom: Tertroxin¤; Triiodothyronine Injection; United States: Cytomel; Triostat; Venezuela: Tertroxin¤; Multi-ingredient Preparations (Latest modification: 20-Apr-2011) The symbol ¤ denotes a preparation which is discontinued or no longer actively marketed. Argentina: Eutroid; Levotrin; Tresite F; Austria: Combithyrex; Novothyral; Prothyrid¤; Belgium: Novothyral; Brazil: Levotiroxina¤; Tyroplus¤; Chile: Martindale: The Complete Drug Reference Novothyral; Czech Republic: Novothyral¤; Thyreotom; France: Euthyral; Germany: FegaCoren N¤; NeyNormin (Revitorgan-Lingual Nr 65)¤; NeyNormin N (Revitorgan-Dilutionen N Nr 65)¤; NeyTumorin (RevitorganLingual Nr 66)¤; NeyTumorin N (Revitorgan-Dilutionen N Nr 66)¤; Novothyral; Prothyrid; Thyreotom¤; Greece: Dithyron; Italy: Tiroide Amsa; Mexico: Cynoplus; Novotiral; Proloid S¤; Redotex; Poland: Novothyral; Russia: Novothyral (Новотирал); Thyreocomb (Тиреокомб); Thyreotom (Тиреотом); South Africa: Diotroxin; Switzerland: Novothyral; Turkey: Bitiron; United States: Euthroid¤; Thyrolar; Pharmacopoeial Preparations (Latest modification: 09-Apr-2011) BP 2011: Liothyronine Tablets; USP 34: Liothyronine Sodium Tablets; Liotrix Tablets; Homoeopathic Preparations (Latest modification: 19-Apr-2011) The symbol ¤ denotes a preparation which is discontinued or no longer actively marketed. Germany: AntiFocal N¤; AntiFocal¤; NeyDil 66N (Revitorgan-Dilutionen Nr. 66 N)¤; NeyGeront (Revitorgan-Lingual Nr 64)¤; NeyGeront N (RevitorganDilutionen N Nr 64)¤; NeyGeront Vitalkapsein A; NeyGeront-Vitalkapseln¤; NeyLing (Revitorgan-Lingual Nr.66)¤;