* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
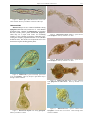
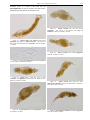
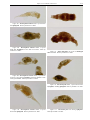
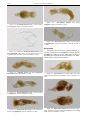
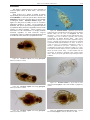
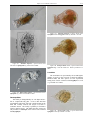
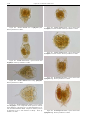
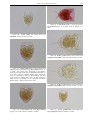
Download CHAPTER 4-6 INVERTEBRATES: ROTIFER
Survey
Document related concepts
Transcript
Glime, J. M. 2013. Invertebrates: Rotifer Taxa. Chapt. 4-6. In: Glime, J. M. Bryophyte Ecology. Volume 2. Bryological Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 6 July 2013 and available at <www.bryoecol.mtu.edu>. 4-6-1 CHAPTER 4-6 INVERTEBRATES: ROTIFER TAXA TABLE OF CONTENTS Taxa on Bryophytes ............................................................................................................................................ 4-6-2 CLASS BDELLOIDEA ...................................................................................................................................... 4-6-6 Adinetidae .................................................................................................................................................... 4-6-6 Habrotrochidae............................................................................................................................................. 4-6-7 Philodinidae ................................................................................................................................................. 4-6-8 CLASS MONOGONONTA ............................................................................................................................. 4-6-10 Order Collothecacea................................................................................................................................... 4-6-10 Collothecidae ...................................................................................................................................... 4-6-10 Order Flosculariacea .................................................................................................................................. 4-6-13 Conochilidae ....................................................................................................................................... 4-6-13 Filiniidae ............................................................................................................................................. 4-6-13 Flosculariidae...................................................................................................................................... 4-6-14 Hexarthriidae ...................................................................................................................................... 4-6-15 Testudinellidae.................................................................................................................................... 4-6-16 Order Ploimida........................................................................................................................................... 4-6-17 Brachionidae ....................................................................................................................................... 4-6-18 Dicranophoridae.................................................................................................................................. 4-6-20 Epiphanidae ........................................................................................................................................ 4-6-24 Euchlanidae......................................................................................................................................... 4-6-25 Gastropodidae ..................................................................................................................................... 4-6-27 Lecanidae............................................................................................................................................ 4-6-27 Ituridae................................................................................................................................................ 4-6-34 Summary ........................................................................................................................................................... 4-6-34 Acknowledgments............................................................................................................................................. 4-6-34 Literature Cited ................................................................................................................................................. 4-6-35 4-6-2 Chapter 4-6: Invertebrates: Rotifer Taxa CHAPTER 4-6 INVERTEBRATES: ROTIFER TAXA Figure 1. Rotifer on a Sphagnum leaf. Photo by Marek Miś at <http://www.mismicrophoto.com/>. Taxa on Bryophytes With about 2200 species, rotifers are a group with a wide range of aquatic, marine, and limnoterrestrial species, permitting us to analyze habitat relations. This is not true with respect to bryophytes because few studies describe those in the bryophyte habitat, and those that do typically simply indicate "moss." This is demonstrated by the delineation of rotifer habitats in the comprehensive study on the relationship of rotifers to habitat, using only macrophytes (housing periphytic rotifers), open water (with planktonic forms), minerogenous sediments (with psammon and hyporheos), organogenous sediments, and other organisms (i.e. parasites and epizoans) (Pejler 1995). Bryophytes are not given separate attention. Pejler (1995) pointed out that rotifers are mostly cosmopolitan, hence suggesting that ecological barriers are more important in determining their distribution. Nevertheless, Pejler considers rotifers to lack strong restrictions of habitat. Extreme environments do support few species, but large numbers of individuals, typically primary consumers. On the other hand, when rotifer species are numerous the differences in their morphology are so great that patterns of adaptations are difficult to define. The few adaptations that do exist include protection from predation among planktonic species. Differences in structure of the trophus seem to facilitate differences in food type. Even in extreme environments, the differences don't seem to correlate with the habitat and the closest relatives seem to occur in "normal" habitats. Pejler considered that adaptations to chemical and physical environments may develop rapidly in geologic time, whereas those changes that are more fundamental occur over a longer time period. It is this group where changes in trophi are most apparent. Although many taxa can be found on bryophytes (Table 1), few have been studied relative to bryophytes, and finding the existing studies among published literature Chapter 4-6: Invertebrates: Rotifer Taxa can be a bit hit or miss. I am unable to summarize adaptations except to suggest that being small (which applies to the entire phylum) and being able to attach may be advantages. The trophi need to be adapted to the available food, with detritus being abundant among the bryophytes. The list provided here is not intended to be comprehensive and the ecological information included 4-6-3 with the images is very incomplete. Likewise, the distribution of species is poorly known, although many are considered cosmopolitan. I have indicated cosmopolitan where I found references so-stating, but I have rarely been able to ascertain countries or distributions and thus have not included those. Due to these limitations, these chapters are organized by classification rather than ecology. Table 1. Species and genera of rotifers known from collections of bryophytes or in bog pools. Authors indicate those who have reported the rotifer species in a collection of bryophytes or from a Sphagnum pool. Those indicated by * indicate those species that have been collected on Sphagnum; + indicates that those collected from Sphagnum were also collected from other bryophytes. If no superscript is given, the author/collector simply said moss. An indication of bog refers to a Sphagnum bog, but not necessarily on a moss (and possibly not a true bog). Please note that some, perhaps most, of the rotifers in this list may not be true bryophyte dwellers, but rather occasional visitors. Those species that have been found in more than one location in association with bryophytes have the species name in bold as this may be an indication it is more than an occasional visitor. Nomenclature follows Segers 2007. Adineta barbata* – S. subsecundum Horkan 1931; Hingley 1993; Jersabek et al. 2003 Adineta gracilis* Horkan 1931; Hingley 1993; Jersabek et al. 2003 Adineta steineri Hirschfelder et al. 1993 Adineta vaga Hingley 1993 Adineta tuberculosa Horkan 1931 Albertia naidis* – Fontinalis Pejler & Bērziņš 1993; Jersabek et al. 2003 Anuraeopsis fissa Horkan 1931 Aspelta angusta* – Fontinalis Pejler & Bērziņš 1993; Jersabek et al. 2003 Aspelta aper*+ – Fontinalis Pejler & Bērziņš 1993 Aspelta beltista* Jersabek et al. 2003 Aspelta chorista* Jersabek et al. 2003 Aspelta circinator* Horkan 1931; Hingley 1993; – Fontinalis Pejler & Bērziņš 1993; Jersabek et al. 2003 Brachionus urceolaris Hingley 1993 Bradyscela clauda Madaliński 1961 Bryceella perpusilla – terrestrial mosses Wilts et al. 2010 Bryceella stylata* Hingley 1993 Bryceella tenella* Hingley 1993; Jersabek et al. 2003 Bryceella voigti Hingley 1993 Callidena symbiotica Hudson 1889 Cephalodella anebodica – bogs Błedzki & Ellison 2003 Cephalodella apocoela* Hingley 1993; Jersabek et al. 2003 Cephalodella auriculata Hingley 1993 Cephalodella belone Jersabek et al. 2003 Cephalodella biungulata* Jersabek et al. 2003 Cephalodella catellina Horkan 1931; Hingley 1993 Cephalodella compressa Jersabek et al. 2003 Cephalodella dorseyi – Fontinalis Jersabek et al. 2003 Cephalodella elegans* Jersabek et al. 2003 Cephalodella eva Horkan 1931 Cephalodella exigua Jersabek et al. 2003 Cephalodella forficula Horkan 1931; Hingley 1993 Cephalodella gibba* Horkan 1931; Hingley 1993; De Smet 2001; Jersabek et al. 2003 Cephalodella gracilis Madaliński 1961 Cephalodella hoodii Horkan 1931 Cephalodella inquilina Jersabek et al. 2003 Cephalodella intuta Hingley 1993 Cephalodella lepida – bog Jersabek et al. 2003 Cephalodella licinia* Jersabek et al. 2003 Cephalodella lipara Jersabek et al. 2003 Cephalodella megalotrocha Horkan 1931 Cephalodella mira* Jersabek et al. 2003 Cephalodella mucronata* Jersabek et al. 2003 Cephalodella nana Hingley 1993 Jersabek et al. 2003 Cephalodella nelitis* Cephalodella pheloma Hingley 1993 Cephalodella physalis – bog Hingley 1993; Jersabek et al. 2003 Cephalodella rostrum Hingley 1993 Cephalodella sterea Horkan 1931 Cephalodella subsecundum Jersabek et al. 2003 Cephalodella tachyphora Jersabek et al. 2003 Cephalodella tantilla Hingley 1993 Cephalodella tantilloides Hingley 1993 Cephalodella ventripes Hingley 1993 Ceratotrocha cornigera Horkan 1931; Hingley 1993 Collotheca ambigua – sessile on Sphagnum Hingley 1993 Collotheca annulata – sessile on Sphagnum Hingley 1993 Collotheca calva – sessile on Sphagnum Hingley 1993 Collotheca campanulata – sessile on Sphagnum Hingley 1993 Collotheca catellina Jersabek et al. 2003 Collotheca coronetta – sessile on Sphagnum Hingley 1993 Collotheca crateriformis* Jersabek et al. 2003 Collotheca heptabrachiata Edmondson 1940 Collotheca hoodii – sessile on Sphagnum Hingley 1993 Collotheca ornata – sessile on Sphagnum Hingley 1993 Collotheca quadrinodosa – sessile on Sphagnum Hingley 1993 Collotheca spinata – sessile on Sphagnum Hingley 1993 Collotheca trilobata – sessile on Sphagnum Hingley 1993 Colurella adriatica Horkan 1931; Hingley 1993 Colurella colurus Madaliński 1961 Colurella hindenburgi* – S. subsecundum Jersabek et al. 2003 Colurella obtusa Horkan 1931; Hingley 1993 Colurella obtusa clausa – bogs Błedzki & Ellison 2003 Colurella paludosa Hingley 1993 Colurella tessellata – bogs Horkan 1931; Hingley 1993; Jersabek et al. 2003 Conochilus – sessile on Sphagnum Hingley 1993 Cyrtonia tuba Horkan 1931 Dicranophorus alcinus* Jersabek et al. 2003 Dicranophorus artamus* Jersabek et al. 2003 Dicranophorus biastis* Jersabek et al. 2003 Dicranophorus capucinus* Jersabek et al. 2003 Dicranophorus colastes* Jersabek et al. 2003 Dicranophorus corystis* Jersabek et al. 2003 Dicranophorus edestes – Fontinalis Jersabek et al. 2003 Dicranophorus epicharus* Pejler & Bērziņš 1993 Dicranophorus facinus* Jersabek et al. 2003 Dicranophorus forcipatus Horkan 1931; Fontinalis Pejler & Bērziņš 1993 Dicranophorus haueri – Fontinalis Pejler & Bērziņš 1993 Dicranophorus hercules Hingley 1993 Dicranophorus isothes* Jersabek et al. 2003 Dicranophorus lenapensis – Fontinalis Jersabek et al. 2003 Dicranophorus longidactylum Hingley 1993 Dicranophorus luetkeni* Hingley 1993; Pejler & Bērziņš 1993; Jersabek et al. 2003 Dicranophorus robustus Hingley 1993; Pejler & Bērziņš 1993 Dicranophorus robusta europaeus – Fontinalis Pejler & Bērziņš 1993 Dicranophorus rostratus* Hingley 1993; Jersabek et al. 2003 Dicranophorus saevus* Jersabek et al. 2003 4-6-4 Chapter 4-6: Invertebrates: Rotifer Taxa Dicranophorus spiculatus - Fontinalis Jersabek et al. 2003 Dicranophorus thysanus – Sphagnum bog & pond Jersabek et al. 2003 Dicranophorus uncinatus Horkan 1931; Hingley 1993; Fontinalis Pejler & Bērziņš 1993 Didymodactylos Ricci & Melone 2000 Dipleuchanis paludosa Hingley 1993 Dipleuchanis propatula Hingley 1993 Dissotrocha aculeata* Horkan 1931; Hingley 1993; Jersabek et al. 2003 Dissotrocha macrostyla Horkan 1931; Hingley 1993 Dissotrocha spinosa Hingley 1993 Dorria dalecarlica – submerged moss on rock Jersabek et al. 2003 Elosa worrallii* Hingley 1993 Encentrum aquilus – Sphagnum ditch Jersabek et al. 2003 Encentrum arvicola* Pejler & Bērziņš 1993 Encentrum carlini* Jersabek et al. 2003 Encentrum elongatum* Pejler & Bērziņš 1993 Encetrum eurycephalum – Fontinalis Pejler & Bērziņš 1993 Encentrum felis* Hingley 1993; Jersabek et al. 2003 Encetrum fluviatile – Fontinalis Pejler & Bērziņš 1993 Encentrum glaucum Hingley 1993 Encentrum incisum* Pejler & Bērziņš 1993 Encetrum lupus* – Fontinalis Pejler & Bērziņš 1993 Encentrum mustela Hingley 1993; Fontinalis Pejler & Bērziņš 1993 Encentrum sutor* Pejler & Bērziņš 1993 Encentrum sutoroides* Pejler & Bērziņš 1993 Encentrum tobyhannaensis* Jersabek et al. 2003 Encentrum tyrphos* Pejler & Bērziņš 1993 Enteroplea lacustris* Horkan 1931; Jersabek et al. 2003 Eosphora ehrenbergi Horkan 1931 Eosphora najas Madaliński 1961 Eothinia elongata Horkan 1931 Euchlanis callysta* Jersabek et al. 2003 Euchlanis calpidia* Jersabek et al. 2003 Euchlanis dilatata Jersabek et al. 2003 Euchlanis incisa Hingley 1993 Euchlanis meneta Hingley 1993 Euchlanis parva Hingley 1993 Euchlanis proxima Hingley 1993 Euchlanis pyriformis Horkan 1931 Euchlanis triquetra – Sphagnum bog Hingley 1993; Jersabek et al. 2003 Euchlanis triquetra subsp pellucida Jersabek et al. 2003 Filinia longiseta Horkan 1931 Filinia terminalis – Sphagnum bog Hingley 1993; Jersabek et al. 2003 Floscularia conifera – sessile on Sphagnum Hingley 1993 Gastropus hyptopus Horkan 1931; Hingley 1993 Gastropus minor – Sphagnum bog Hingley 1993; Jersabek et al. 2003 Habrotrocha ampulla* Jersabek et al. 2003 Habrotrocha angusticollis* Hingley 1993 Habrotrocha aspera Horkan 1931 Habrotrocha bidens Hingley 1993 Habrotrocha collaris Horkan 1931; Hingley 1993 Habrotrocha constricta Horkan 1931; Hingley 1993 Habrotrocha elegans Hingley 1993 Habrotrocha eremita Peters et al. 1993 Habrotrocha flava Hirschfelder et al. 1993 Habrotrocha fusca Hirschfelder et al. 1993 Habrotrocha insignis Hirschfelder et al. 1993 Habrotrocha lata* Horkan 1931; Hingley 1993; Jersabek et al. 2003 Habrotrocha longula Hingley 1993 Habrotrocha microcephala Madaliński 1961 Habrotrocha milnei Hingley 1993 Habrotrocha minuta Hingley 1993 Horkan 1931 Horkan 1931 Hingley 1993; Jersabek et al. 2003 Habrotrocha roeperi* Horkan 1931; Hingley 1993 Habrotrocha rosa Madaliński 1961 Habrotrocha tridens Madaliński 1961 Hexarthra mira Horkan 1931 Itura aurita Horkan 1931 Kellicottia longispina Madaliński 1961 Keratella mixta* Jersabek et al. 2003 Keratella quadrata Hingley 1993 Keratella serrulata* Bērziņš & Pejler 1987; Hingley 1993 Lecane agilis* Hingley 1993; Jersabek et al. 2003 Lecane calcaria* Jersabek et al. 2003 Lecane clara Hingley 1993 Lecane climacois Jersabek et al. 2003 Lecane closterocerca Hingley 1993 Lecane cornuta Hingley 1993 Lecane curvicornis acronyrha - Sphagnum bog Jersabek et al. 2003 Lecane depressa – Sphagnum bog Hingley 1993; Jersabek et al. 2003 Lecane elasma Jersabek et al. 2003 Lecane flexilis Hingley 1993; Riccia fluitans Jersabek et al. 2003 Lecane galeata* – Sphagnum bog Hingley 1993; S. subsecundum Jersabek et al. 2003 Lecane gallagherorum* Jersabek et al. 2003 Lecane hamata Hingley 1993 Lecane inermis* Hingley 1993; Jersabek et al. 2003 Lecane lauterborni Jersabek et al. 2003 Lecane ligona Jersabek et al. 2003 Lecane lunaris Madaliński 1961; Hingley 1993 Lecane mira* Jersabek et al. 2003 Lecane mitis* Jersabek et al. 2003 Lecane pertica Jersabek et al. 2003 Lecane pyrrha* – Sphagnum bog Hingley 1993; Jersabek et al. 2003 Lecane rhopalura – submerged moss Jersabek et al. 2003 Lecane quadridentata Horkan 1931 Lecane satyrus* Jersabek et al. 2003 Lecane scutata Koste & Shiel 1990 Lecane signifera* Hingley 1993; Jersabek et al. 2003 Lecane signifera ploenensis* Hingley 1993; Jersabek et al. 2003 Lecane stichaea* Hingley 1993; Jersabek et al. 2003 Lecane subulata Jersabek et al. 2003 Lecane tenuiseta* Jersabek et al. 2003 Lecane thalera* Jersabek et al. 2003 Lecane tryphema – Sphagnum bog Jersabek et al. 2003 Lecane ungulata Madaliński 1961 Lepadella acuminata Hingley 1993 Lepadella akrobeles* Jersabek et al. 2003 Lepadella bractea* Jersabek et al. 2003 Lepadella eurysterna – Fontinalis novae-angliae Jersabek et al. 2003 Lepadella ovalis Hingley 1993 Lepadella patella Hingley 1993 Lepadella pterygoidea* Jersabek et al. 2003 Lepadella pterygoides Hingley 1993 Lepadella triba* Hingley 1993; Jersabek et al. 2003 Lepadella triptera Horkan 1931; Hingley 1993 Lepadella venefica* – emersed S. subsecundum; Sphagnum bog Jersabek et al. 2003 Lindia annecta de Manuel Barrabin 2000 Lindia torulosa Hingley 1993 Macrochaetus collinsi Hingley 1993 Macrochaetus multispinosus* Jersabek et al. 2003 Macrotrachela bilfingeri Madaliński 1961 Macrotrachela concinna Hingley 1993 Habrotrocha pulchra Habrotrocha pusilla Habrotrocha reclusa* – S. subsecundum Chapter 4-6: Invertebrates: Rotifer Taxa Peters et al. 1993; Jersabek et al. 2003 Macrotrachela habita* Horkan 1931; Hirschfelder et al. 1993; Jersabek et al. 2003 Macrotrachela insolita Hirschfelder et al. 1993 Macrotrachela multispinosa Horkan 1931; Hingley 1993 Sphagnum bog; on "tree moss" Jersabek et al. 2003 Macrotrachela muricata Horkan 1931 Macrotrachela musculosa Hirschfelder et al. 1993 Macrotrachela nana Madaliński 1961 Macrotrachela papillosa Horkan 1931; Hingley 1993 Macrotrachela plicata Horkan 1931; Hingley 1993; on "tree moss" Jersabek et al. 2003 Macrotrachela punctata Hirschfelder et al. 1993 Macrotrachela quadricornifera* Horkan 1931; Hingley 1993; Jersabek et al. 2003 Macrotrachela zickendrahti* Jersabek et al. 2003 Mikrodides chalaena Horkan 1931 Microcodon clavus* Horkan 1931; Hingley 1993; Jersabek et al. 2003 Mniobia incrassata Hingley 1993 Mniobia magna Hingley 1993 Mniobia obtuscicornis Hingley 1993 Mniobia orta Peters et al. 1993 Mniobia russeola Horkan 1931; Hirschfelder et al. 1993 Mniobia scarlatina – "tree moss" Jersabek et al. 2003 Mniobia symbiotica Horkan 1931; Hingley 1993 Mniobia tetraodon Horkan 1931 Monommata actices* Hingley 1993; Jersabek et al. 2003 Monommata aequalis Horkan 1931 Monommata aeschyna* Hingley 1993 Monommata astia Hingley 1993 Monommata hyalina* Jersabek et al. 2003 Monommata longiseta Hingley 1993 Monommata maculata Hingley 1993 Monommata phoxa Hingley 1993 Mytilina macrocera* Jersabek et al. 2003 Mytilina mucronata Horkan 1931; Hingley 1993 Mytilina ventralis var. brevispina Hingley 1993 Notommata allantois Hingley 1993 Notommata brachyota Horkan 1931 Notommata cerberus Horkan 1931; Hingley 1993 Notommata cherada – Sphagnum bog Jersabek et al. 2003 Notommata contorta Hingley 1993 Sphagnum pool Jersabek et al. 2003 Notommata copeus Horkan 1931; Hingley 1993 Notommata cyrtopus Horkan 1931 Notommata falcinella* Hingley 1993; Sphagnum subsecundum Jersabek et al. 2003 Notommata fasciola* Jersabek et al. 2003 Notommata groenlandica Hingley 1993 Notommata pachyura Horkan 1931; Hingley 1993 Notommata saccigera Hingley 1993 Notommata tripus Horkan 1931; Hingley 1993 Otostephanos macrantennus Ricci 1998 Otostephanos regalis Hirschfelder et al. 1993 Otostephanos torquatus Peters et al. 1993 Paracolurella aemula* Jersabek et al. 2003 Paracolurella logima* Jersabek et al. 2003 Pedipartia gracilis* Jersabek et al. 2003 Philodina acuticornis Hingley 1993 Philodina brevipes Hingley 1993 Philodina citrina Hirschfelder et al. 1993; Sphagnum bog; "tree moss" Jersabek et al. 2003 Philodina erythrophthalma Horkan 1931 Philodina flaviceps Horkan 1931; Madaliński 1961 Philodina nemoralis Hingley 1993 Philodina plena* Hirschfelder et al. 1993; Jersabek et al. 2003 Philodina roseola Hirschfelder et al. 1993 Macrotrachela ehrenbergii* 4-6-5 Philodina rugosa Horkan 1931; Hingley 1993 Philodina vorax Hirschfelder et al. 1993 Philodinavus paradoxus Madaliński 1961 Pleurata chalcicodis Jersabek et al. 2003 Pleurata tithasa Jersabek et al. 2003 Pleurata vernalis Jersabek et al. 2003 Pleuretra brycei Madaliński 1961 Pleuretra lineata Hirschfelder et al. 1993 Pleurotrocha robusta – Sphagnum bog Jersabek et al. 2003 Ploesoma lynceus Hingley 1993 Polyarthra euryptera Horkan 1931 Polyarthra minor* Hingley 1993 Polyarthra vulgaris Hingley 1993 Proales cognita* – S. cuspidatum Jersabek et al. 2003 Proales decipiens Horkan 1931; Hingley 1993 Proales doliaris – Sphagnum bog Hingley 1993; Jersabek et al. 2003 Proales fallaciosa Hingley 1993 Proales latrunculus Hingley 1993 Proales micropus Hingley 1993 Proales minima Hingley 1993 Proales palimmeka* – submerged Jersabek et al. 2003 Proales sordida* Horkan 1931 Proales theodora Madaliński 1961 Proalinopsis caudatus Horkan 1931; Hingley 1993 Proalinopsis gracilis – Riccia fluitans Jersabek et al. 2003 Proalinopsis squamipes Hingley 1993; Sphagnum ditch Jersabek et al. 2003 Pseudoploesoma formosum Jersabek et al. 2003 Ptygura brachiata – sessile on Sphagnum Hingley 1993 Ptygura cristata Edmondson 1940 Ptygura crystallina Horkan 1931 Ptygura elata Hingley 1993 Ptygura longicornis – sessile on Sphagnum Hingley 1993 Ptygura longipes – sessile on Sphagnum Hingley 1993 Ptygura melicerta Horkan 1931 Ptygura pilula – sessile on Sphagnum Hingley 1993 Ptygura rotifer – sessile on Sphagnum Hingley 1993 Ptygura velata – sessile on Sphagnum Hingley 1993 Resticula melandocus Hingley 1993 Resticula nyssa Hingley 1993 Rotaria haptica Hingley 1993 Rotaria macroceros Horkan 1931 Rotaria macrura Horkan 1931; Hingley 1993 Rotaria magna-calcarata Hingley 1993 Rotaria neptunoida Hingley 1993 Rotaria quadrioculata Hingley 1993 Rotaria rotatoria Horkan 1931; Madaliński 1961 Rotaria socialis Hingley 1993 Rotaria sordida Horkan 1931; Hirschfelder et al. 1993 Rotaria spicata Hingley 1993 Rotaria tardigrada Hingley 1993 Scaridium longicaudum Horkan 1931 Scepanotrocha rubra Horkan 1931; Hingley 1993 Squatinella bifurca* Jersabek et al. 2003 Squatinella longispinata Hingley 1993; Sphagnum bog Jersabek et al. 2003 Squatinella microdactyla Hingley 1993 Squatinella mutica Hingley 1993 Squatinella rostrum (formerly S. mutica) Hingley 1993 Squatinella retrospina* – Sphagnum bog Jersabek et al. 2003 Squatinella tridentata Hingley 1993 Stephanoceros fimbriatus – sessile on Sphagnum Hingley 1993 Stephanoceros millsii Hingley 1993 Streptognatha lepta* Hingley 1993; Jersabek et al. 2003 Synchaeta pectinata Horkan 1931; Hingley 1993 Synchaeta tremula Horkan 1931 Taphrocampa annulosa Hingley 1993 Taphrocampa clavigera* Hingley 1993; Jersabek et al. 2003 Testudinella clypeata Horkan 1931 4-6-6 Chapter 4-6: Invertebrates: Rotifer Taxa Testudinella emarginula Hingley 1993 Testudinella epicopta – Sphagnum bog Jersabek et al. 2003 Testudinella incisa emarginula – Sphagnum bog Jersabek et al. 2003 Testudinella patina Hingley 1993 Tetrasiphon hydrocora* Norgrady 1980; Hingley 1993 Trichocerca brachyura Horkan 1931 Trichocerca bicristata Horkan 1931; Hingley 1993 Trichocerca cavia Hingley 1993 Trichocerca collaris Hingley 1993 Trichocerca elongata Hingley 1993 Trichocerca harveyensis – Fontinalis disticha Jersabek et al. 2003 Hingley 1993 Trichocerca junctipes Trichocerca lata* Jersabek et al. 2003 Trichocerca longiseta Hingley 1993 Trichocerca ornata – Sphagnum bog Jersabek et al. 2003 Jersabek et al. 2003 Trichocerca parvula* Trichocerca platessa* Jersabek et al. 2003 Trichocerca porcellus Hingley 1993; Fontinalis Jersabek et al. 2003 CLASS BDELLOIDEA Trichocerca rattus Horkan 1931; Hingley 1993; Sphagnum bog Jersabek et al. 2003 Trichocerca rosea* in bog Hingley 1993; Jersabek et al. 2003 Trichocerca rossae* Jersabek et al. 2003 Trichocerca rotundata* Jersabek et al. 2003 Trichocerca scipi* Jersabek et al. 2003 Trichocerca similis Horkan 1931 Trichocerca tenuior Horkan 1931; Jersabek et al. 2003 Trichocerca tigris Horkan 1931; Hingley 1993: Sphagnum and Riccia in pond Jersabek et al. 2003 Trichotria cornuta Jersabek et al. 2003 Trichotria pocillum Horkan 1931; Hingley 1993 Trichotria similis Jersabek et al. 2003 Trichotria tetractis* Horkan 1931; Hingley 1993; Sphagnum in bog Jersabek et al. 2003 Jersabek et al. 2003 Trichotria tetractis caudatus Trichotria truncata Horkan 1931; Hingley 1993 Wierzejskiella elongata* Jersabek et al. 2003 Wierzejskiella velox* Hingley 1993; Pejler & Bērziņš 1993; Jersabek et al. 2003 demonstrated by DNA and a diversity of narrow ecological niches (Fontaneto et al. 2011). This class of rotifers is exclusively parthenogenetic (giving from unfertilized eggs), negating the need for males to complete the life cycle. This group is comprised of ~460 species, only one of which is marine (Segers 2008). They are distinguished from the Monogononta by the presence of two ovaries (Monogononta have only one). The bdelloids are known from freshwater and soil, and are common on bryophytes. They have a retractable head with a well-developed corona that is divided into two parts. Movement includes both swimming and crawling, but they seldom venture into the plankton (Fontaneto & Ricci 2004). Crawling is similar to the movement of inchworms, or some leeches. The name Bdelloidea is derived from the Greek word meaning leeches, referring to this method of movement. Most of the bdelloids survive unfavorable periods, particularly drought, by entering a type of dormancy known as anhydrobiosis (Gilbert 1974; Ricci 1987, 1998, 2001). It is this ability, along with their parthenogenetic reproduction (no male is needed) (Ricci 1992) that fosters their cosmopolitan distribution (Fontaneto et al. 2006b, 2007, 2008b). And this may also be the reason that Horkan (1931), in his report on Irish rotifers, found only this group on mosses other than those in bogs. Furthermore, no Bdelloidea were present in the Irish bogs, on bog moss, or in bog pools, suggesting they may need those dry periods. Only one carnivorous bdelloid is known, and it is not known from bryophytes. Rather, the bdelloids filter or scrape or browse their diet of bacteria, one-celled algae, yeast, or particulate organic matter (Ricci 1984). Figure 3. Lüth. Adinetidae Ricci and Covino (2005) demonstrated various aspects of anhydrobiosis in this family, using Adineta ricciae. Rotifers that recovered from anhydrobiosis had similar longevity and significantly higher fecundity than did the hydrated controls. Lines of offspring produced after the anhydrobiosis dormancy likewise had significantly higher fecundity and longevity than controls from mothers of the same age. The genus Adineta has many cryptic species, as Figure 4. Adineta gracilis, a species known from Sphagnum and other mosses. Photo by Jersabek et al. 2003. Figure 2. Adineta barbata female, a species known to live on Sphagnum subsecundum (Figure 3) and other mosses. Photo by Jersabek et al. 2003. Sphagnum subsecundum. Photo by Michael Chapter 4-6: Invertebrates: Rotifer Taxa 4-6-7 Figure 5. Adineta vaga, a moss dweller that is 0.2-0.3 mm when extended. Photo by Jean-Marie Cavanihac at Micscape. Habrotrochidae Habrotrocha species are common inhabitants among Sphagnum (Bateman 1987; Peterson et al. 1997; Błedzki & Ellison 1998). Habitats for Habrotrocha, in particular H. rosa, include pitcher plants (Sarracenia purpurea), where they are a major food source for co-habiting members of the Culicidae (mosquitoes) (Bateman 1987), causing the mosquito population numbers to rise (Błedzki & Ellison 1998). The rotifers are an important source of N and P in the bog/fen-dwelling pitcher plants. Figure 8. Habrotrocha collaris female, a species known from bryophytes. Photo by Jersabek et al. 2003. Figure 9. Habrotrocha constricta female, a species known from bryophytes. Photo by Jersabek et al. 2003. Figure 6. Habrotrocha, a genus with many species that occur on bryophytes. Photo by Proyecto Agua Water Project through Creative Commons. Figure 10. Habrotrocha lata female, a species collected from Sphagnum and other mosses. Photo by Jersabek et al. 2003. Figure 7. Habrotrocha ampulla from among Sphagnum. Photo by Jersabek et al. 2003. Figure 11. Habrotrocha lata, a species collected from bryophytes in more than one location. Photo through EOL Creative Commons. 4-6-8 Chapter 4-6: Invertebrates: Rotifer Taxa Philodinidae The philodinids use their cilia or foot and proboscis (Figure 26) to facilitate swimming (Hickernell 1917). At high temperatures these rotifers engage in active swimming, but in cold water they creep like a leech with the cilia retracted. During feeding, they attach themselves by the foot and use the cilia to direct food to the pharynx. When drying occurs, the animal forms a ball and dries up. The ball is formed by retracting both the head and the foot into the trunk of the rotifer and losing all the water, pulling the organs together and eliminating spaces. When they get water again, they resume their normal shape in ten minutes or less. Figure 12. Dissotrocha aculeata female, a species known from Sphagnum and other mosses. Photo by Jersabek et al. 2003. Figure 13. Dissotrocha macrostyla subsp. tuberculata female, a species known from bryophytes in more than one location. Photo by Jersabek et al. 2003. Figure 14. Macrotrachela ehrenbergii female, a species known from Sphagnum. Photo by Jersabek et al. 2003. Figure 15. Macrotrachela habita female, a species known from Sphagnum and other mosses. Photo by Jersabek et al. 2003. Figure 16. Macrotrachela multispinosa female, a species known from "tree moss" and other mosses. Photo by Jersabek et al. 2003. Figure 17. Macrotrachela multispinosa from among "tree moss." Photo by Jersabek et al. 2003. Figure 18. Macrotrachela plicata, a species known from "tree moss" and other mosses. Photo by Jersabek et al. 2003. Chapter 4-6: Invertebrates: Rotifer Taxa 4-6-9 Figure 23. Philodina plena female, a species known from Sphagnum. Photo by Jersabek et al. 2003. Figure 19. Macrotrachela quadricornifera female, a species known from Sphagnum and other mosses. Photo by Jersabek et al. 2003. Figure 24. Philodina roseola, a species known to inhabit bryophytes. Photo by Proyecto Agua Water Project through Creative Commons. Figure 20. Macrotrachela sp., a genus with a number of species that live on Sphagnum. Photo by Walter Pfliegler. Figure 25. Philodina roseola females with eggs, a species known to inhabit bryophytes. Photo by Jersabek et al. 2003. Figure 21. Mniobia scarlatina from among "tree moss." Photo by Jersabek et al. 2003. Figure 22. Philodina citrina female, a species known from Sphagnum bogs and "tree moss." Photo by Jersabek et al. 2003. Figure 26. Rotaria macroceros, known from bog pools. The genus Rotaria is able to move among mosses and other substrata by creeping with its head and foot (van Egmond 1999). The foot (Figure 27) is sticky, enabling it to attach to a surface while it feeds (Dickson & Mercer 1966; Schmid-Araya 1998). The anterior cilia (Figure 28) make a current that directs the food toward the pharynx for ingestion. Note the proboscis. Photo from GLERL NOAA website. 4-6-10 Chapter 4-6: Invertebrates: Rotifer Taxa in fresh water of limnoterrestrial habitats (Segers 2008). It differs from the Bdelloidea in having two sexes and having only one ovary. Nevertheless, asexual reproduction occurs over and over until environmental conditions, often related to crowding, trigger the reproduction to become sexual (Welch 2008). At this time, the eggs of the amictic (nonsexual) females hatch into mictic females that produce their eggs by meiosis. The haploid eggs that are not fertilized develop into much smaller males and fertilization of a female by these males produces diploid eggs that become resting eggs. The monogonont rotifers mostly eat small particles and organisms by filtering them, some actually seize them, and some are parasitic. Figure 27. Rotaria macrura from among Sphagnum and other mosses, showing fully extended foot. Photo by Jersabek et al. 2003. Figure 28. Rotaria, showing the two wheels that direct the food into the gullet. Photo by Yuuji Tsukii. Figure 29. Rotaria rotatoria female, a species known from bryophytes in more than one location. Photo by Jersabek et al. 2003. Order Collothecacea Many members of this order are sessile (attached) and some are colonial. These rotifers have a foot that lacks toes, but they possess many foot glands that are used for adhesion. The females are predominantly sessile, but males and immature rotifers are free-living.. The rotary apparatus surrounds a funnel-like invagination. Many are surrounded with a jelly sheath. Collothecidae Many members of the Collothecidae are plant and algal inhabitants. Collotheca gracilipes, a plant inhabitant, is selective in its location on its substrate (Wallace & Edmondson 1986). On plants such as Elodea canadensis, it selected (98%) the lower (abaxial) surfaces of the leaves. When given equal opportunities for four plant species, it selected Lemna minor over Elodea canadensis, but in the field more were found on Elodea canadensis, with densities reaching more than six individuals per mm2. Light made a difference, with 91% of the rotifers selecting the adaxial surface in continuous light, but showing no preference in continuous darkness. Alpha amylase appears to be the chemical that helps them to identify a plant substrate. Those rotifers that were induced to settle on the abaxial surface produced more eggs than those that were induced to settle on the adaxial (upper) surface. It would be interesting to see if these relationships persist on liverworts like Riccia fluitans (Figure 31) and Ricciocarpos natans. But what would they do on mosses like Fontinalis? Figure 30. Rotaria, fully extended as it would be for its leech-like movement. This is a genus with several bryophytedwelling species that can move about the bryophytes in this manner. Photo by Wim van Egmond. CLASS MONOGONONTA This is the largest of the two classes of rotifers, comprised of ~1570 species, ~1488 of which are free-living Figure 31. Riccia fluitans, a substrate for Lecane flexilis and other rotifer species, stranded here above water. Photo by Janice Glime. Chapter 4-6: Invertebrates: Rotifer Taxa 4-6-11 The Collothecidae provide us with evidence of adaptive strategies embodied in reproduction. An examination of 65 species of rotifers, including this family, revealed that egg volume of rotifers increased as body volume increased, but the relative size of eggs actually decreased as body size increased (Wallace et al. 1998). This means that smaller species, typical among planktonic species, invest the most in egg production. The Flosculariidae species are of intermediate size and their relative investment in egg mass is likewise intermediate. The Collothecidae family has the largest species and the lowest relative biomass of egg production among those examined by Wallace et al. Figure 34. Collotheca campanulata, a species that is known as sessile on Sphagnum in bogs and occurs in bog pools. Photo by Jersabek et al. 2003. Figure 32. Collotheca, a common genus on Sphagnum. Photo by Proyecto Agua Water Project through Creative Commons. Figure 35. Collotheca campanulata, a species that is known as sessile on Sphagnum and occurs in bog pools. Photo by Yuuji Tsukii. Figure 36. Collotheca catellina, a species known from bryophytes. Photo by Jersabek et al. 2003. Figure 33. Collotheca sp., a common genus on Sphagnum. Photo by Ed Purp through Micrographia. Figure 37. Collotheca catellina, a species known from bryophytes. Photo by Jersabek et al. 2003. 4-6-12 Chapter 4-6: Invertebrates: Rotifer Taxa Figure 42. Collotheca trilobata from among Sphagnum. Photo by Jersabek et al. 2003. Figure 38. Collotheca coronetta, a species that occurs sessile on Sphagnum. Photo by Jersabek et al. 2003. Figure 39. Collotheca crateriformis Sphagnum. Photo by Jersabek et al. 2003. from among Figure 40. Collotheca crateriformis Sphagnum. Photo by Jersabek et al. 2003. from among Figure 43. Stephanoceros fimbriatus, a sessile species that can occur ln Sphagnum. Photo by Wim van Egmond. Figure 44. Stephanoceros fimbriatus female, a species that occurs sessile on Sphagnum. Photo by Jersabek et al. 2003. Figure 41. Collotheca ornata, a species that lives in bogs and is sessile on Sphagnum. Photo by Jersabek et al. 2003. Figure 45. Stephanoceros millsii, a species known from bryophytes. Note the eggs. Photo by Jersabek et al. 2003. Chapter 4-6: Invertebrates: Rotifer Taxa 4-6-13 Order Flosculariacea Not only do the members of this order lack toes; some of the planktonic species lack feet as well. Nevertheless, they have multiple foot glands to secrete glue. The rotary organ has a double ring of cilia that surrounds the anterior of its lobe-like appendages. Species may be either freeliving or sessile and are suspension feeders. Conochilidae This family, or at least Conochilus hippocrepis (Figure 46, Figure 48), is sensitive to increasing predator pressure from the copepod Parabroteas sarsi (Diéguez & Balseiro 1998). As the predator increases in size and begins to prey on the Conochilus hippocrepis, this rotifer responds by increasing its colony size (Figure 47). The only members of this family that seem to be known as bryophyte associates are found among Sphagnum. Figure 46. Conochilus hippocrepis subsp. unicornis female, member of a genus known to associate with Sphagnum. The species Conochilus hippocrepis is typically planktonic in both ponds and large bodies of water, generally with a pH of 6.3-8.3 and temperature range of 6.4-15.4°C (de Manuel Barrabin 2000). Its colonies can reach 30-60 members that are joined in a gelatinous case. It eats detritus and bacteria (Pourriot 1977). Photo by Jersabek et al. 2003. Figure 47. Conochilus sp. colony. This genus has species that are sessile on Sphagnum. Photo by Wim van Egmond. Figure 48. Conochilus hippocrepis female, member of a genus known on Sphagnum. Photo by Jersabek et al. 2003. Filiniidae Only two members of the Filinidae seem to be known from bryophytes: Filinia longiseta (Figure 49-Figure 50) and F. terminalis (Figure 51). The latter lake species is morphologically variable but seems to occupy a narrow and well defined niche (Ruttner-Kolisko 1980). It prefers temperatures below 12-15°C. At an oxygen content of less than 2 mg L-1, it can reach as many as 1000 individuals per liter. Not surprisingly, it is facultatively anaerobic. Its food sources include bacteria that are chemosynthetic or decompose plankton. The members Filiniidae are highly variable and likely comprise a number of microspecies (Ruttner-Kolisko 1989). This is at least in part due to the parthenogenetic reproduction that can quickly lead to a clone of genetically identical individuals in a founder population in a lake or other habitat. This is furthermore complicated by the absence of many good morphological characters by which to distinguish species. In the Filinia terminalis-longiseta group, ecological properties differ and suggest the existence of these microspecies, or perhaps species. Figure 49. Filinia longiseta is known from bryophytes in England and Ireland. This is typically a cosmopolitan planktonic species of lakes, ponds, moorland waters, and even brackish water (de Manuel Barrabin 2000). It lives in a wide range of warm temperatures (7.7-26.2°C) and pH (6.3-9.9). It is a filter feeder on detritus, bacteria, and small algae like Chlorella in a size range of 10-12 µm (Pourriot 1965) and most likely competes for its food with members of the rotifer genus Conochilus. Photo by Jersabek et al. 2003. 4-6-14 Chapter 4-6: Invertebrates: Rotifer Taxa Figure 50. Filinia longiseta from bryophytes in a pond in Pennsylvania, USA. This species is also known from bog pools. Photo by Jersabek et al. 2003. Figure 53. Floscularia conifera female, a species that occurs sessile on Sphagnum and in bog pools. Photo by Jersabek et al. 2003. Figure 51. Filinia terminalis female, a cosmopolitan, planktonic species known from bryophytes and Sphagnum bogs (de Manuel Barrabin 2000). Its preferred conditions are mesotrophic to eutrophic in a pH range of 6.64-8.22. Its temperature range is relatively wide: 7.3-22.8°C, although de Manuel Barrabin considers it to be a species of the cool hypolimnion. Photo by Jersabek et al. 2003. Flosculariidae In this family the male is small and free-swimming, whereas the female lives in a tube and usually attaches by its modified foot. Some of these females (e.g. Ptygura linguata) live on the bladders of species of the bladderwort Utricularia. But, sadly for the rotifers, they also constitute part of the diet of these same bladderworts (Mette et al. 2000). This habitat affords the rotifers a special aid in getting food as it is sucked into the bladder. Bryophytes can offer no such aid, and although the genera on bryophytes are often the same because they are sessile, species differ. As I read through account after account of rotifer sampling, I couldn't help but wonder if more attention should be given to the bryophyte habitat for locating new rotifer species, especially for sessile groups like this one. Figure 54. Ptygura, a common genus on bryophytes, showing its feeding cilia. Photo by Micrographia. Figure 55. Ptygura sp with the green alga Spirogyra. Photo from Micrographia. Figure 52. Ptygura, a genus with a number of species known to be sessile on Sphagnum, feeding among algae. Photo by Micrographia. Figure 56. Ptygura brachiata female, known to be sessile on Sphagnum. Photo by Jersabek et al. 2003. Chapter 4-6: Invertebrates: Rotifer Taxa Figure 57. Ptygura brachiata female, a species known to be sessile on Sphagnum. Photo by Jersabek et al. 2003. 4-6-15 Figure 61. Ptygura melicerta colony in a lake in Wisconsin, USA. This species is known from bryophytes and bog pools. Photo by Jersabek et al. 2003. Figure 58. Ptygura crystallina female from the Pocono Mountains, Pennsylvania, USA. This species has been collected with bryophytes and can occur in bogs. Photo by Jersabek et al. 2003. Figure 62. Ptygura pilula female sessile on a Sphagnum leaf; it also occurs in bog pools. Photo by Jersabek et al. 2003. Figure 59. Ptygura melicerta colony in a lake in Wisconsin, USA. This species can occur among bryophytes and in bog pools. Photo by Jersabek et al. 2003. Figure 63. Ptygura rotifer female, a species known to occur sessile on Sphagnum. Photo by Jersabek et al. 2003. Figure 60. Ptygura melicerta female from a lake in Connecticut, USA. Here it is among Cyanobacteria; it can occur among bryophytes. Photo by Jersabek et al. 2003. Hexarthriidae In a study of a Turkish lake, Gülle et al. (2010) found that rotifers were most abundant from June through August 4-6-16 Chapter 4-6: Invertebrates: Rotifer Taxa and disappeared from November through April. It was a member of the Hexarthriidae, Hexarthra fennica that was one of the dominant taxa – 51% of the zooplankton. The rotifers were most dense at a depth of 5 m. Figure 66. Testudinella sp, a genus that occurs on bryophytes. Note the complete retraction of the foot. Photo by Wim van Egmond. Figure 64. Hexarthra mira female from Mexico. This species is known from bryophytes and from bogs. Photo by Jersabek et al. 2003. Figure 67. Testudinella clypeata, color modified. This species is known from bryophytes and can occur in bogs. Photo by Leasi Francesca through EOL. Figure 65. Hexarthra mira female from Mexico. This species is known from bryophytes and from bogs. Photo by Jersabek et al. 2003. Testudinellidae The family Testudinellidae includes both salt water and fresh water species. It is characterized by having dorsal and ventral plates of the lorica that are completely fused laterally. The body is greatly flattened dorsiventrally. The foot is long and retractile (see Figure 68 and Figure 73) with a tuft of cilia at its tip. These rotifers are free-swimming, typically in the littoral zone, but members of Testudinella may also occur on bryophytes and in Sphagnum pools as well as on other macrophytes. There are three genera, but only Testudinella seems to be represented on bryophytes. Figure 68. Testudinella epicopta from among Sphagnum. Photo by Jersabek et al. 2003. Chapter 4-6: Invertebrates: Rotifer Taxa Figure 69. Testudinella emarginula from a Sphagnum bog. This cosmopolitan species lives on plant surfaces, although it occasionally occurs in the plankton (de Manuel Barrabin 2000). It is a cold water species (7.7-7.8°C) with a circumneutral pH preference (pH 6..8-7.5) and wide alkalinity range. Photo by Jersabek et al. 2003. Figure 70. Testudinella incisa emarginula subsp emarginula from a Sphagnum bog. Photo by Jersabek et al. 2003. 4-6-17 Figure 72. Testudinella patina; some members of this genus are Antarctic moss dwellers. Photo by Yuuji Tsukii. Figure 73. Testudinella tridentata subsp dicella from among Sphagnum. Photo by Jersabek et al. 2003. Figure 74. Testudinella tridentata subsp dicella from among Sphagnum. Photo by Jersabek et al. 2003. Figure 71. Testudinella patina female. This is a planktonic species that likes small bodies of water where the aquatic plants are abundant (de Manuel Barrabin 2000). Bryophytes are among the aquatic plants in some associations where it has been found. The aquatic plant area provides it with its preferred foods of the green alga Chlorella and diatoms. It tolerates high salinity and lives in a pH range of 6.3-8.89. It enjoys a wide temperature range of 9.5-24.3°C. Photo by Jersabek et al. 2003. Order Ploimida This order has the most families. But are these species ones likely to be on bryophytes? Wallace et al. (2008) asked if "everything is everywhere?" They answered this question in the Chihuahua Desert pools in Mexico. They found that indeed the specialized, warm-water habitat of 4-6-18 Chapter 4-6: Invertebrates: Rotifer Taxa the desert did not support "everything." The fauna was dominated by families that are also common on bryophytes: Brachionidae, Lecanidae, Lepadellidae, and Notommatidae. Both habitats dry up. Brachionidae This is a family dominated by planktonic species and was the family with the most species represented in Spanish reservoirs (de Manuel Barrabin 2000), but a few seem to spend time among bryophytes, perhaps as a place to avoid predation, or just dropped there by moving water. An interesting study by Stenson (1982) demonstrated, however, that an experimental reduction of the fish population led to an increase in larger rotifers and a decrease in the smaller filter-feeding species such as Keratella cochlearis, a member of the Brachionidae. Stenson attributed this to a change in competition for food from rotifers such as Polyarthra (Figure 75). Figure 75. Polyarthra major, a large rotifer that eats smaller rotifers. Note the feather-like blades that are used like paddles in swimming. Photo by Wim van Egmond. Figure 77. Anuraeopsis fissa from a pond in Pennsylvania, USA. Photo by Jersabek et al. 2003. Figure 78. Anuraeopsis fissa showing a single, lightsensitive red eyespot and cilia. Photo from GLERL at plingfactory. Feeding rates are inversely related to the density of food organisms in Keratella cochlearis, as well as in Polyarthra vulgaris and Polyarthra dolichoptera (Bogdan & Gilbert 1982). Keratella preferred Chlamydomonas to all other foods offered, perhaps explaining its rarity among mosses, where Chlamydomonas also is rare. Figure 79. Brachionus urceolaris, a planktonic species that is common in small, alkaline bodies of water (pH 7.25-9) (de Manuel Barrabin 2000). It can occur in moving water and is relatively tolerant of high salinity. It is a cosmopolitan species with a wide temperature tolerance (7.35-24.3°C). Despite its alkaline preference, Hingley (1993) found it closely associated with Sphagnum in a bog. Photo from Smithsonian Institution. Figure 76. Anuraeopsis fissa from a pond in Pennsylvania, USA. This is a planktonic rotifer that has been found among bryophytes and in bog pools. It prefers warm water and a eutrophic habitat (Margalef 1955). It frequents small water bodies (de Manuel Barrabin 2000). Its food includes bacteria and detritus (Pourriot 1977) and it may become food for the rotifer Asplanchna (Guiset 1977). Photo by Jersabek et al. 2003. Brachionus urceolaris, and probably others, has a survival trick against predation. The eggs survived consumption by predators such as the cladoceran Leptodora kindtii without harm (Nagata et al. 2011). Often the cladocerans would eject the eggs, and they typically ejected the lorica while digesting the living contents. There Chapter 4-6: Invertebrates: Rotifer Taxa 4-6-19 was a negative correlation between the portion of unconsumed (ejected) eggs and the length of the predator. Nevertheless, hatching success seemed to be independent of the predator's body length. As many as 75% of the undigested eggs hatched successfully. Figure 83. Kellicottia longispina demonstrating spines that may help in attaching it to bryophytes (Madaliński 1961). Photos GLERL at plingfactory. Figure 80. Brachionus urceolaris, a planktonic species that can occur in a Sphagnum bog. Photo by Michael Verolet. Figure 81. Kellicottia longispina female, a central European species known from bryophytes, is actually a planktonic species. Its long spines no doubt help to protect it from predation. It is active year-round as an inhabitant of oligotrophic lakes with a rather narrow pH range of 8.2-8.5, but as expected its temperature range is broad (10.6-21.8°C) and it does not occur in small bodies of water (de Manuel Barrabin 2000). Its food is primarily chrysomonads and centric diatoms (Pourriot 1977). Photo by Jersabek et al. 2003. Figure 82. Kellicottia longispina demonstrating spines that may help in attaching it to bryophytes (Madaliński 1961). Photos GLERL at plingfactory. Figure 84. Keratella mixta from among Sphagnum. Photo by Jersabek et al. 2003. Figure 85. Keratella quadrata female, a species known from bryophytes. This is also a cosmopolitan species that is active year-round (de Manuel Barrabin 2000). It is tolerant of mineralization and survives a wide pH range of 6.64-10.19. Its temperature range is likewise wide (6.4-26.1°C), as expected for a perennial species. It has broad food preferences, including detritus, bacteria, and algae in the Chlorococcales, Volvocales, Euglenales, Chrysophyceae, and diatoms (Pourriot 1977). Photo by Jersabek et al. 2003. 4-6-20 Chapter 4-6: Invertebrates: Rotifer Taxa Figure 86. Keratella quadrata female, a species known from bryophytes. Photo by Jersabek et al. 2003. Figure planktonic particularly 1987). Its 18.6°C (de 2003. 87. Keratella serrulata female. This is the only brachionid that is a specialist of acid water, water from bogs with Sphagnum (Bērziņš & Pejler known pH is around 6.6 and temperature around Manuel Barrabin 2000). Photo by Jersabek et al. Figure 88. Keratella serrulata feeds on algae in the Chrysophyceae and Volvocales (Pourriot 1977). It lives in acid water, especially the outflow of Sphagnum bogs and poor fens. Photo from GLERL at plingfactory. Figure 89. Keratella serrulata showing rotary cilia. Photo from GLERL at plingfactory. Figure 90. Keratella serrulata female, a species known from Sphagnum bogs and poor fen waters. Photo by Jersabek et al. 2003. Dicranophoridae The Dicranophoridae are predators and are agile in pursuing and capturing their prey (Pejler & Bērziņš 1993). Unlike many rotifers, the Dicranophoridae are not planktonic – other predatory rotifers exist there – and they avoid the sediments where their prey organisms are not sufficiently abundant. Unlike many rotifers, these have been documented on two species of bryophytes through a study of their substrata. Albertia naidis, Aspelta angusta, A. aper, A. circinator, Dicranophorus forcipatus, D. haueri, D. robusta europaeus, D. uncinatus, Encetrum eurycephalum, E. fluviatile, E. lupus, and E. mustela were all present on 1-10% of the 122 collections of Fontinalis. Aspelta aper, A. circinator, Dicranophorus epicharus, D. luetkeni, Encetrum arvicola, E. elongatum, E. incisum, E. lupus, E. sutor, E. sutoroides, E. tyrphos, and Wierzejsklella velox were all present on 1-10% of the 194 collections of Sphagnum. Both sets of bryophyte dwellers occurred on a wide variety of plant substrata – none were specific to bryophytes. Whereas some families of rotifers are active yearround, the Dicranophoridae are apparently sensitive to warm weather. In a study of those members that live in the Chapter 4-6: Invertebrates: Rotifer Taxa 4-6-21 interstitial spaces of a beach of the North Sea, the Dicranophoridae can only be found in the cold seasons, disappearing in mid-summer (Tzschaschel 1983). Figure 95. Aspelta circinator side view from among Sphagnum. This species is also known from bogs and Fontinalis. Photo by Jersabek et al. 2003. Figure 91. Albertia naidis subsp intrusor from among Sphagnum and parasitic on Stylaria lacustris. This species is also known from the aquatic moss Fontinalis. Photo by Jersabek et al. 2003. Figure 96. Aspelta circinator from among Sphagnum. Photo by Jersabek et al. 2003. Figure 92. Trophus of Aspelta angusta from among mosses on rock. Photo by Jersabek et al. 2003. Figure 93. Aspelta aper, a rotifer that occurs on both Fontinalis and Sphagnum species (Pejler & Bērziņš 1993). Photo by Jersabek et al. 2003. Figure 97. Aspelta chorista from among the moss Warnstorfia exannulata (formerly Drepanocladus exannulatus). Photo by Jersabek et al. 2003. Figure 94. Aspelta beltista from among Sphagnum. Photo by Jersabek et al. 2003. Figure 98. Dicranophorus alcimus from among Sphagnum. Photo by Jersabek et al. 2003. 4-6-22 Figure 99. Dicranophorus artamus Sphagnum. Photo by Jersabek et al. 2003. Chapter 4-6: Invertebrates: Rotifer Taxa from among Figure 100. Dicranophorus biastis from among Sphagnum. Photo by Jersabek et al. 2003. Figure 104. Dicranophorus forcipatus, a rotifer found among bryophytes in several studies. Upper Photo from the Smithsonian Institution, lower from GLERL NOAA. Figure 101. Dicranophorus capucinus from among Sphagnum. Photo by Jersabek et al. 2003. Figure 105. Dicranophorus hercules capucinoides female, a species known from bryophytes. Photo by Jersabek et al. 2003. Figure 102. Dicranophorus capucinus from among Sphagnum. Photo by Jersabek et al. 2003. Figure 103. Dicranophorus colastes Sphagnum. Photo by Jersabek et al. 2003. from among Figure 106. Dicranophorus luetkeni female, a species known from Sphagnum. Photo by Jersabek et al. 2003. Chapter 4-6: Invertebrates: Rotifer Taxa 4-6-23 Figure 107. Dicranophorus luetkeni male, a species known from Sphagnum. Photo by Jersabek et al. 2003. Figure 108. Dicranophorus robustus female, a species found with bryophytes in more than one location. Photo by Jersabek et al. 2003. Figure 111. Dorria dalecarlica can occur on submerged moss in streams. Photo by Jersabek et al. 2003. Figure 109. Dicranophorus robustus female, a species that is known to live among bryophytes and ingests members of the rotifer genus Lecane. Photo by Jersabek et al. 2003. Figure 112. Encentrum felis female, a species known from bryophytes, including Sphagnum. Photo by Jersabek et al. 2003. Figure 110. Dicranophorus rostratus female, a species known from Sphagnum. Photo by Jersabek et al. 2003. Figure 113. Encentrum felis from among Sphagnum. Photo by Jersabek et al. 2003. 4-6-24 Chapter 4-6: Invertebrates: Rotifer Taxa Figure 114. Encentrum glaucum female, a species known from bryophytes. Photo by Jersabek et al. 2003. Figure 119. Wierzejskiella elongata Sphagnum. Photo by Jersabek et al. 2003. from among Figure 120. Wierzejskiella velox female, a species known from Sphagnum in more that one location. Photo by Jersabek et al. 2003. Figure 115. Trophus of Encentrum tobyhannaensis from among Sphagnum. Often this is the only structure that can be recognized in old collections. Photo by Jersabek et al. 2003. Figure 116. Pedipartia gracilis from among Sphagnum subsecundum. Photo by Jersabek et al. 2003. Epiphanidae This family has rotifers that are usually planktonic, so like most of the rotifers on bryophytes, it is likely that the bryophyte is a temporary refuge. Many of the members of this family are marine (Koste 1978; Fontaneto et al. 2006a, 2008a), where no bryophytes are known. Figure 121. Cyrtonia tuba from a pond in Ohio, USA. This species has been collected from mosses. Photo by Jersabek et al. 2003. Figure 117. Streptognatha lepta female, a species known from Sphagnum. Photo by Jersabek et al. 2003. Figure 118. Streptognatha lepta female, a rotifer known to associate with Sphagnum. Photo by Jersabek et al. 2003. Figure 122. Mikrocodides chlaena female from New Jersey, USA. This species has been collected from mosses and from bog pools. Photos by Jersabek et al. 2003. Chapter 4-6: Invertebrates: Rotifer Taxa Euchlanidae This family is characterized by a lorica consisting of connected plates (Koste & Shiel 1989). The toes are elongated. There seems to be a paucity of studies on rotifers beyond listing the taxa present in various water bodies. In the Euchlanidae, at least one species that is known from Sphagnum seems to have been the subject of several kinds of biological studies. Euchlanis dilatata (Figure 126Figure 127) has proven its ability to serve as a sensitive biomonitor (Sarmaa et al. 2001). In an experiment on herbicides, this species experienced a significant reduction in population density and rate of population increase in the presence of methyl parathion. These responses were exacerbated as the concentration of methyl parathion increased, regardless of food (Chlorella vulgaris) concentration. However, higher food concentrations served to mediate the effect on the rate of population increase. 4-6-25 Figure 126. Euchlanis dilatata, a planktonic species known from littoral zones of small bodies of eutrophic waters (de Manuel Barrabin 2000), but can occur on bryophytes and other macrophytes. It occurs in both fresh water and brackish water, preferring water rich in nutrients, especially those favoring Cyanobacteria (de Manuel Barrabin 2000). These waters generally have a pH range of 6.3-9.6 and a temperature range of 6.4-24°C. Although only 200 µm long, this species is consumed by damselfly naiads (Ejsmont-Karabin et al. 1993). In the lab, it is able to survive on Cyanobacteria (Oscillatoria redekei, O. limnetica, Aphanizomenon flos-aquae, Anabaena sp.) and a prochlorophyte (Prochlorothrix hollandica) (Gulati et al. 1993). In the field it consumes detritus, bacteria, Cyanobacteria, and the diatom Cyclotella (Carlin 1943). It often benefits from the convenience of attaching to planktonic algae colonies (Pejler 1962). Photo by Proyecto Agua Water Project through Creative Commons. Figure 123. Euchlanis callysta from among Sphagnum. Photo by Jersabek et al. 2003. Figure 124. Euchlanis calpidia from among Sphagnum. Photo by Jersabek et al. 2003. Figure 125. Euchlanis calpidia from among Sphagnum. Photo by Jersabek et al. 2003. Figure 127. Euchlanis dilatata, a species that has been collected from bryophytes. Photo from GLERL at plingfactory website. Figure 128. Euchlanis incisa mucronata female, a species known from bryophytes. Photo by Jersabek et al. 2003. 4-6-26 Chapter 4-6: Invertebrates: Rotifer Taxa Figure 129. Euchlanis incisa, a species known from bryophytes. Photo from GLERL and plingfactory website. Figure 133. Euchlanis parva female, a species known from bryophytes. Photo by Jersabek et al. 2003. Figure 134. Euchlanis triquetra from among Sphagnum. Photo by Jersabek et al. 2003. Figure 130. Euchlanis incisa mucronata female, a species known from bryophytes. Photo by Jersabek et al. 2003. Figure 131. Euchlanis meneta female, a species known from bryophytes. Photo by Jersabek et al. 2003. Figure 132. Euchlanis meneta female, a species known from bryophytes. Photo by Jersabek et al. 2003. Figure 135. Euchlanis triquetra from among Sphagnum. Photo by Jersabek et al. 2003. Figure 136. Euchlanis triquetra subsp pellucida from among Sphagnum. Photo by Jersabek et al. 2003. Chapter 4-6: Invertebrates: Rotifer Taxa 4-6-27 Figure 139. Gastropus hyptopus, a species known from bryophytes and from bog pools. Photo by Jersabek et al. 2003. Figure 137. Euchlanis triquetra, a species known from more than one Sphagnum bog. Photos from GLERL. Figure 140. Gastropus minor female, a species known from Sphagnum bogs. Note the ventral foot. Photo by Jersabek et al. 2003. Lecanidae The Lecanidae were represented by the second highest number of species in the reservoirs in Spain (de Manuel Barrabin 2000) and their species are well represented among those rotifers collected with bryophytes as well (e.g. Jersabek et al. 2003). Figure 138. Mikrocodides chlaena, a species known from a Sphagnum bog. Photo from GLERL website. Gastropodidae This family is distinguished by its oval shape and saclike or compressed body plan. It has a thin shell that surrounds the entire body with only a small opening for the head and ventrally located foot (Figure 139) that is sometimes absent. The family is primarily in freshwater with few marine species. The family has nine genera, but only members of Gastropus seem to have been collected from bryophytes. Figure 141. Lecane agilis female, a species known from Sphagnum. Photo by Jersabek et al. 2003. 4-6-28 Chapter 4-6: Invertebrates: Rotifer Taxa Figure 142. Lecane calcaria from a Sphagnum pond. Photo by Jersabek et al. 2003. Figure 146. Lecane cornuta female, a species known from bryophytes, with foot extended. Photo by Jersabek et al. 2003. Figure 147. Lecane cornuta female, a species known from bryophytes, with foot retracted. Photo by Jersabek et al. 2003. Figure 143. Lecane clara female, a species known from bryophytes. Photo by Jersabek et al. 2003. Figure 148. Lecane depressa female, a species known from Sphagnum bogs. Photo by Jersabek et al. 2003. Figure 144. Lecane climacois from among Sphagnum. Photo by Jersabek et al. 2003. Figure 145. Lecane closterocerca female, a species known from bryophytes. This cosmopolitan littoral species is common in the plankton in a pH range of 6.7-9.1 and temperatures of 7.824°C (de Manuel Barrabin 2000). Despite its common presence in freshwater, it has a wide tolerance of salinity. Photo by Jersabek et al. 2003. Figure 149. Lecane depressa female, a species known from Sphagnum bogs. Photo by Jersabek et al. 2003. Chapter 4-6: Invertebrates: Rotifer Taxa 4-6-29 Figure 153. Lecane flexilis female, a species known from Riccia fluitans (Figure 31) in ponds. Photo by Jersabek et al. 2003. Figure 150. Lecane elasma from among mosses and Sphagnum. Photo by Jersabek et al. 2003. Figure 154. Lecane cf galeata female, a species known from Sphagnum subsecundum in bogs. Photo by Jersabek et al. 2003. Figure 151. Lecane flexilis female, a species known from bogs and from the thallose liverwort Riccia fluitans (Figure 31) in ponds. This species occurs infrequently in the plankton, preferring instead the littoral zone (de Manuel Barrabin 2000). It occurs more frequently in alkaline habitats (Pejler 1962; Koste 1978) in a pH range of 6.64-7.87, although Koste and Shiel (1990) found it in slightly acidic water. Its wide temperature range (9.50-21.13°C) permits it to be cosmopolitan (de Manuel Barrabin 2000). (Photo by Jersabek et al. 2003. Figure 155. Lecane cf galeata female, a species known from Sphagnum subsecundum in bogs. Photo by Jersabek et al. 2003. Figure 152. Lecane flexilis from among Riccia fluitans (Figure 31) in a pond. Photo by Jersabek et al. 2003. Figure 156. Lecane gallagherorum subsp copeis from among Sphagnum. Photo by Jersabek et al. 2003. 4-6-30 Chapter 4-6: Invertebrates: Rotifer Taxa Figure 157. Lecane gallagherorum subsp psammophila from among Sphagnum. Photo by Jersabek et al. 2003. Figure 161. Lecane lauterborni from among Sphagnum wheeleri in Hawaii. Photo by Jersabek et al. 2003. Figure 158. Lecane hamata female, a cosmopolitan, littoral species living on plant substrata and known from bryophytes (de Manuel Barrabin 2000). It occurs at pH levels around 7.9 with a is known from a temperature range of 11.9-13.5. Photo by Jersabek et al. 2003. Figure 159. Lecane inermis female, a species known from Sphagnum. Typically a littoral species, it occurs in warm water such as thermal springs and geysers (de Manuel Barrabin 2000). Its typical temperature is around 19.4°C, but it can be found near geysers at temperatures up to 62.5°C. Its environmental pH is usually around 7.3. Photo by Jersabek et al. 2003. Figure 160. Lecane inermis female, a species known from Sphagnum. Photo by Jersabek et al. 2003. Figure 162. Lecane ligona from a Sphagnum pool. Photo by Jersabek et al. 2003. Figure 163. Lecane lunaris female, a cosmopolitan littoral species that is frequent in the plankton (de Manuel Barrabin 2000) and is known to inhabit bryophytes. It is known from water that is rich in nutrients with a pH of 6.3-9.2 and a temperature range of 7.2-26.2°C (de Manuel Barrabin 2000). Photo by Jersabek et al. 2003. Figure 164. Lecane lunaris female, a species known to inhabit bryophytes. Photo by Jersabek et al. 2003. Chapter 4-6: Invertebrates: Rotifer Taxa 4-6-31 Figure 169. Lecane mitis subsp depressa from among Sphagnum. Photo by Jersabek et al. 2003. Figure 165. Lecane lunaris female, a species known to inhabit bryophytes. Photo by Jersabek et al. 2003. Figure 166. Side view of Lecane lunaris, a rotifer collected from bryophytes in more than one locality. Photo from Proyecto Agua. Figure 170. Lecane pertica from among Sphagnum. Photo by Jersabek et al. 2003. Figure 167. Lecane mira from among Sphagnum. This cosmopolitan species lives on aquatic plants and is common in somewhat acid waters, but can also be common at a pH around 7.2. It is known from a temperature around 10.8°C. Photo by Jersabek et al. 2003. Figure 168. Lecane mitis from among Sphagnum. Photo by Jersabek et al. 2003. Figure 171. Lecane pertica from among Sphagnum. Photo by Jersabek et al. 2003. Figure 172. Lecane pyrrha female, a species known from Sphagnum bogs. Photo by Jersabek et al. 2003. 4-6-32 Chapter 4-6: Invertebrates: Rotifer Taxa Figure 173. Lecane quadridentata from a lake in Pennsylvania, USA. This species has been collected from bryophytes and from bog pools. Photo by Jersabek et al. 2003. Figure 176. Lecane scutata from a canal in Florida, USA. This species occurs in the littoral zone of lakes where it lives on plant surfaces (de Manuel Barrabin 2000). It is an acidophile, commonly living among Sphagnum (Koste & Shiel 1990), but it is cosmopolitan and probably not restricted to strongly acid habitats (de Manuel Barrabin 2000). Photo by Jersabek et al. 2003. Figure 177. Lecane signifera female, a species known to live among Sphagnum. Photo by Jersabek et al. 2003. Figure 174. Lecane rhopalura from among submerged moss in a pond. Photo by Jersabek et al. 2003. Figure 175. Lecane satyrus from among Sphagnum. Photo by Jersabek et al. 2003. Figure 178. Lecane signifera ploenensis from among Sphagnum. Photo by Jersabek et al. 2003. Chapter 4-6: Invertebrates: Rotifer Taxa Figure 179. Lecane stichaea female, a species known from among Sphagnum. Photo by Jersabek et al. 2003. 4-6-33 Figure 183. This Lecane tenuiseta was collected from among Sphagnum (Jersabek et al. 2003). It is typically a littoral species, known from a pH around 7.9 and a temperature around 13.5°C (de Manuel Barrabin 2000). Although it is cosmopolitan, its restricted habitat needs make it relatively rare. Photo by Jersabek et al. 2003. Figure 180. Lecane stichaea female, a rotifer associated with Sphagnum in more than one location. Photo by Jersabek et al. 2003. Figure 184. Lecane thalera from among Sphagnum. Photo by Jersabek et al. 2003. Figure 181. Lecane subulata from among Sphagnum. Photo by Jersabek et al. 2003. Figure 185. Lecane tryphema in a Sphagnum bog. Photo by Jersabek et al. 2003. Figure 182. Lecane subulata from among Sphagnum. Photo by Jersabek et al. 2003. Figure 186. Lecane ungulata female, a species known to inhabit bryophytes. Photo by Jersabek et al. 2003. 4-6-34 Chapter 4-6: Invertebrates: Rotifer Taxa one species, Itura aurita, that had been collected from mosses. Figure 187. Lecane ungulata female, a species known to inhabit bryophytes. Photo by Jersabek et al. 2003. Figure 190. Itura aurita female from Pocono Lake, Pennsylvania, USA. This species is known from bryophytes and from bogs. Photo by Jersabek et al. 2003. Summary Figure 188. Lecane ungulata female, a species known to inhabit bryophytes. Photo by Jersabek et al. 2003. The rotifers in Bdelloidea are often represented on bryophytes. They include three families known from bryophytes: Adinetidae, Habrotrochidae, Philodinidae. The Adinetidae have three species known from bryophytes. The Habrotrochidae have a number of species from bogs and from bryophytes. The Philodinidae creep in cold water and live attached on plants; a number of species occur on bryophytes. The Class Monogononta have three orders and are the largest class of rotifers. Many members of order Collothecacea are sessile. Some members of Collothecidae are known from Riccia fluitans, Sphagnum, and other bryophytes. The order Flosculariacea are suspension feeders and known bryophyte dwellers include members of Conochilidae, Filiniidae, Flosculariidae, Hexarthriidae, and Testudinellidae. The order Ploimida includes both planktonic and non-planktonic families that are known from bryophytes: Brachionidae, Dicranophoridae, Epiphanidae, Euchlanidae, Gastropodidae, Lecanidae, and Ituridae. Additional families are in the next sub-chapter. Acknowledgments Figure 189. Lecane ungulata tenuior female, a species known to inhabit bryophytes. Photo by Jersabek et al. 2003. Ituridae This small family seems to have little written about it beyond species lists and taxonomic distinctions. Even the map of its distribution showed nothing. I could find only Bryonetters have been wonderful in making their photographs available to me and seeking photographs from others. Tom Powers and Walter Dioni helped me obtain images and permission from others. C. D. Jersabek very generously gave me permission to use the wealth of images from the Online Catalog of Rotifers. Tom Thekathyil and Des Callahan helped me in finding and gaining permission from Marek Mís for the beautiful image in the frontispiece and others. Many photographers have been generous with permission for the use of their images and others have provided them online through Creative Commons and other public domain sources. Chapter 4-6: Invertebrates: Rotifer Taxa Literature Cited Bateman, L. E. 1987. A bdelloid rotifer living as an inquiline in leaves of the pitcher plant, Sarracenia purpurea. Hydrobiologia 147: 129-133. Bērziņš, B. and Pejler, B. 1987. Rotifer occurrence in relation to pH. Hydrobiologia 147: 107-116. Błedzki, L. A. and Ellison, A. M. 1998. Population growth and production of Habrotrocha rosa Donner (Rotifera: Bdelloidea) and its contribution to the nutrient supply of its host, the northern pitcher plant, Sarracenia purpurea L. (Sarraceniaceae). Hydrobiologia 385: 193-200. Błedzki, L. A. and Ellison, A. M. 2003. Diversity of rotifers from northeastern U.S.A. bogs with new species records for North America and New England. Hydrobiologia 497: 5362. Bogdan, K. G. and Gilbert J. J. 1982. Seasonal patterns of feeding by natural populations of Keratella, Polyarthra, and Bosmina: Clearance rates, selectivities, and contributions to community grazing. Limnol. Oceanogr 27: 918-934. Carlin, B. 1943. Die Planktonrotatorien des Motalastrom. Medd. Lunds Univ. Limnol. Inst. 5: l-256. Cavanihac, J.-M. 2004. The fascinating world of rotifers. based on March 2004 edition of Micscape Magazine. Accessed 25 January 2012 at <http://www.microscopyuk.org.uk/mag/artmar04/jmcrotif.html>. Dickson, M. R. and Mercer, E. H. 1966. Fine structure of the pedal gland of Philodina roseola (Rotifer). J. Microsc. 5: 81-90. Diéguez, M. and Balseiro, E. 1998. Colony size in Conochilus hippocrepis: Defensive adaptation to predator size. Hydrobiologia 387/388: 421-425. Edmondson, W. T. 1940. The sessile Rotatoria of Wisconsin. Trans. Amer. Microsc. Soc. 59: 433-459. Egmond, W. van. 1999. Gallery of Rotifers. Accessed 6 May 2012 at <http://www.microscopy-uk.org.uk>. Ejsmont-Karabin, J., Siewertsen, K., and Gulati, R. D. 1993. Changes in size, biomass, and production of Euchlanis dilatata lucksiana Hauer during its lifespan. Hydrobiologia 255/256: 77-80. Fontaneto, D. and Ricci, C. 2004. Rotifera: Bdelloidea. In: Yule, C. M. and Yong, H. S. (eds.). Freshwater Invertebrates of the Malaysian Region. Academy of Sciences Malaysia, Kuala Lumpur, Malaysia, pp. 121-126. Fontaneto, D., Barraclough, T. G., Chen, K., Ricci, C., and Herniou, E. A. 2008a. Molecular evidence for broad-scale distributions in bdelloid rotifers: Everything is not everywhere but most things are very widespread. Molec. Ecol. 17: 3136-3146. Fontaneto, D., Smet, W. H. De, and Melone G. 2008b. Identification key to the genera of marine rotifers worldwide. Meiofauna Marina 16. Fontaneto, D., Smet, W. H. De, and Ricci, C. 2006a. Rotifers in saltwater environments, re-evaluation of an inconspicuous taxon. J. Marine Biol. Assoc. U. K. 86: 623-656. Fontaneto, D., Ficetola, G. F., Ambrosini, R., and Ricci, C. 2006b. Patterns of diversity in microscopic animals: Are they comparable to those in protists or in larger animals? Global Ecol. Biogeogr. 15: 153-162. Fontaneto, D., Herniou, E. A., Barraclough, T. G., and Ricci, C. 2007. On the global distribution of microscopic animals: New worldwide data on bdelloid rotifers. Zool. Stud. 46: 336-346. Fontaneto, D., Iakovenko, N., Eyres, I., Kaya, M., Wyman, M., and Barraclough, T. G. 2011. Cryptic diversity in the 4-6-35 genus Adineta Hudson & Gosse, 1886 (Rotifera: Bdelloidea: Adinetidae): a DNA taxonomy approach. Hydrobiologia 662: 27-33. Gilbert, J. J. 1974. Dormancy in rotifers. Trans. Amer. Microsc. Soc. 93: 490-513. Guiset, A. 1977. Stomach content in Asplanchna and Ploesoma. Arch. Hydrobiol. Beih. 8: 126-129. Gulati, R. D., Ejsmnt-Karabin, J., and Postema, G. 1993. Feeding in Euchlanis dilatata lucksiana Hauer on filamentous Cyanobacteria and a prochlorophyte. Hydrobiologia 255/256: 269-274. Gülle, I., Turna, I. I., Güçlü, S. S., Gülle, P., and Güçlü, Z. 2010. Zooplankton seasonal abundance and vertical distribution of highly alkaline Lake Burdur, Turkey. Turkish J. Fish. Aquat. Sci. 10: 245-254. Hickernell, L. M. 1917. A study of desiccation in the rotifer, Philodina roseola, with special reference to cytological changes accompanying desiccation. Biol. Bull. 32: 343-407. Hingley, M. 1993. Microscopic Life in Sphagnum. Illustrated by Hayward, P. and Herrett, D. Naturalists' Handbook 20. [iiv]. Richmond Publishing Co. Ltd., Slough, England, 64 pp.. 58 fig. 8 pl. (unpaginated). Hirschfelder, A., Koste, W., and Zucchi, H. 1993. Bdelloid rotifers in aerophytic mosses: Influence of habitat structure and habitat age on species composition. In: Gilbert, J. J., Lubzens, E., and Miracle, M. R. (eds.). 6. International Rotifer Symposium, Banyoles, Spain, 3-8 Jun 1991. Rotifer Symposium VI. Hydrobiologia 255-256: 343-344. Horkan, J. P. K. 1931. A list of Rotatoria known to occur in Ireland, with notes on the habitats and distribution. Irish Fisheries Investigations. Series A (Freshwater) No. 21. Government Publications Sale Office, G.P.O. Arcade, Dublin, 25 pp. Hudson, C. T. 1889. Rotifera and their distribution. Nature 39: 438-441. Jersabek, C. D., Segers, H., and Morris, P. J. 2003. An illustrated online catalog of the Rotifera in the Academy of Natural Sciences of Philadelphia (version 1.0: 2003-April-8). [WWW database]. Accessed 23 February 2012 at <http://rotifer.acnatsci.org/rotifer.php>. Koste, W. 1978. Rotatoria. Die Rädertiere Mitteleuropas (Uberordnung Monogononta) Bestimmungswerk begriindet von Max Voigt. 2 Vols. Borntraeger, Stuttgart, 673 pp., 234 pls. Koste, W. and Shiel, R. J. 1989. Rotifera from Australian inland waters. III. Euchlanidae, Mytilinidae and Trichotriidae (Rotifera: Monogononta). Trans. Royal Soc. Austral. 113: 85-114. Koste, W. and Shiel, R. J. 1990. Rotifera from Australian inland waters V. Lecanidae (Rotifera: Monogononta). Trans. Royal Soc. S. Austral. 114(1): 1-36. Madaliński, K. 1961. Moss dwelling rotifers of Tatra streams. Polskie Arch. Hydrobiol. 9: 243-263. Manuel Barrabin, J. de. 2000. The rotifers of Spanish reservoirs: Ecological, systematical and zoogeographical remarks. Limnetica 19: 91-167. Margalef, R. 1955. Contribución a1 estudio de la fauna de las aguas dulces del Noroeste de Espaiia. P. Inst. Biol. Apl. 21: 137-171. Mette, N., Wilbert, N., and Barthlott, W. 2000. Food composition of aquatic bladderworts (Utricularia, Lentibulariaceae) in various habitats. Beitr. Biol. Pflanzen 72: 1-13. . Nagata, T., Sakamoto, M., Tanaka, Y., and Hanazato, T. 2011. Egg viability of the rotifer Brachionus urceolaris after 4-6-36 Chapter 4-6: Invertebrates: Rotifer Taxa ingestion by the predatory cladoceran Leptodora kindtii. Hydrobiologia 665: 263-266. Norgrady, T. 1980. Canadian rotifers II. Parc Mont Tremblant, Quebec. Hydrobiologia 71: 35-46. Pejler, B. 1962. Taxonomic notes on some planktic fresh-water rotifers. Zool. Bidr: Uppsala 35: 307-319. Pejler, B. 1995. Relation to habitat in rotifers. Hydrobiologia 313/314: 267-278. Pejler, B. and Bērziņš, B. 1993. On choice of substrate and habitat in bdelzoid rotifers. Hydrobiologia 255/256: 333338. Peters, U., Koste, W., and Westheide, W. 1993. A quantitative method to extract moss-dwelling rotifers. Hydrobiologia 255-256: 339-341. Peterson, R. L., Hanley, L., Walsh, E., Hunt, H., and Duffield, R. M. 1997. Occurrence of the rotifer, Habrotrocha cf. rosa Donner, in the purple pitcher plant, Sarracenia purpurea L., (Sarraceniaceae) along the eastern seaboard of North America. Hydrobiologia 354: 63-66. Pourriot, R. 1965. Recherches sur l'ecologie des rotiteres. Vie Milieu 21: 5-181. Pourriot, R. 1977. Food and feeding habits of Rotifera. Arch. Hydrobiol. Beih. 8: 243-260. Ricci, C. 1984. Culturing of some bdelloid rotifers. Hydrobiologia 112: 45-51. Ricci, C. 1987. Ecology of bdelloids: How to be successful. Hydrobiologia 147: 117-127. Ricci, C. 1992. Rotifers: Parthenogenesis and heterogony. In: Dallai, R. (ed.). Sex origin and evolution. Selected Symposia and Monographs U.Z.I., 6, Mucchi, Modena, pp. 329-341. Ricci, C. 1998. Anhydrobiotic capabilities of bdelloid rotifers. Hydrobiologia 387/388: 321-326. Ricci, C. 2001. Dormancy patterns in rotifers. Hydrobiologia 446/447: 1-11. Ricci, C. and Covino, C. 2005. Anhydrobiosis of Adineta ricciae: Costs and benefits. Develop. Hydrol. 181: 307-314. Ricci, C. and Melone, G. 2000. Key to the identification of the genera of bdelloid rotifers. Hydrobiologia 418: 73-80. Ruttner-Kolisko, A. 1980. The abundance and distribution of Filinia terminalis in various types of lakes as related to temperature, oxygen, and food. Hydrobiologia 73: 169-175. Ruttner-Kolisko, A. 1989. Problems in taxonomy of rotifers, exemplified by the Filinia longiseta - terminalis complex. Hydrobiologia 187/188: 291-298. Sarmaa, S. S. S., Nandinia, S., Gama-Floresa, J. L., and Fernandez-Araiza, M. A. 2001. Population growth of Euchlanis dilatata (Rotifera): Combined effects of methyl parathion and food (Chlorella vulgaris). J. Environ. Sci. Health, Part B: Pesticides Food Contam. Agric. Wastes 36: 43-54. Schmid-Araya, J. M. 1998. Rotifers in interstitial sediments. Hydrobiologia 387/388: 231-240. Segers, H. 2007. Annotated checklist of the rotifers (Phylum Rotifera) with notes on nomenclature, taxonomy and distribution. Zootaxa 1564: 1-104. Segers, H. 2008. Global diversity of rotifers (Rotifera) in freshwater. Hydrobiologia 595: 49-59. Smet, W. H. De. 2001. Freshwater Rotifera from plankton of the Kerguelen Islands (Subantarctica). Hydrobiologia 446/447: 261-272. Stenson, J. A. E. 1982. Fish impact on rotifer community structure. Hydrobiologia 87: 57-64. Tzschaschel, G. 1983. Seasonal abundance of psammon rotifers. Hydrobiologia 104: 275-278. Wallace, R. L. and Edmondson, W. T. 1986. Mechanism and adaptive significance of substrate selection by a sessile rotifer. Ecology 67: 314-323. Wallace, R. L., Cipro, J. J., and Grubbs, R. W. 1998. Relative investment in offspring by sessile Rotifera. Hydrobiologia 387/388: 311-316. Wallace, R. L., Walsh, E. J., Schröder, T., Rico-Martínez, R., and Rios-Arana, J. V. 2008. Species composition and distribution of rotifers in Chihuahuan Desert waters of México: Is everything everywhere? Verh. Internat. Verein. Limnol. 30: 73-76. Welch, David Mark. 2008. Monogononta. In Access Science, ©McGraw-Hill Companies. Accessed 9 May at <http://www.accessscience.com>. Wilts, E. F., Martinez Arbizu, P., and Ahlrichs, W. H. 2010. Description of Bryceella perpusilla n. sp. (Monogononta: Proalidae), a new rotifer species from terrestrial mosses, with notes on the ground plan of Bryceella remane, 1929. Internat. Rev. Hydrobiol. 95: 471-481.