* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 5 Crystal field theory
Survey
Document related concepts
Transcript
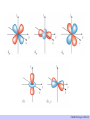
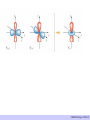
CHAPTER 5: CRYSTAL FIELD THEORY RECALL The elements in the periodic table are often divided into four categories: (1) main group elements, (2) transition metals, (3) lanthanides, and (4) actinides. CHEM210/Chapter 5/2014/01 TRANSITION METALS vs. MAIN-GROUP ELEMENTS There is some controversy about the classification of the elements on the boundary between the main group and transition-metal elements on the right side of the table. The elements in question are zinc (Zn), cadmium (Cd), and mercury (Hg). CHEM210/Chapter 5/2014/02 THE ELECTRON CONFIGURATION OF TRANSITION-METAL IONS The relationship between the electron configurations of transition-metal elements and their ions is complex. EXAMPLE Consider the chemistry of cobalt which forms complexes that contain either Co2+ or Co3+ ions. Co: [Ar] 4s2 3d7 Co2+: [Ar] 3d7 Co3+: [Ar] 3d6 In general, electrons are removed from the valence shell s orbitals before they are removed from valence d orbitals when transition metals are ionized. CHEM210/Chapter 5/2014/03 THE ORIGIN OF COLOUR - ABSORPTION CHEM210/Chapter 5/2014/04 The colour can change depending on a number of factors e.g. • Metal charge • Ligand CHEM210/Chapter 5/2014/05 CHEM210/Chapter 5/2014/06 Are there any simple theories to explain the colours in transition metal complexes? There is a simple electrostatic model used by chemists to rationalize the observed results THIS THEORY IS CALLED CRYSTAL FIELD THEORY It is NOT A RIGOROUS BONDING THEORY but merely a simplistic approach to understanding the possible origins of photo- and electrochemical properties of the transition metal complexes. Method of explaining some physical properties that occur in transition metal complexes. Involves a simple electrostatic argument which can yield reasonable results and predictions about the d orbital interactions in metal complexes. CHEM210/Chapter 5/2014/07 THE OCTAHEDRAL CRYSTAL FIELD Consider metal ion, Mm+, lying at the centre of an octahedral set of point charges. CHEM210/Chapter 5/2014/08 Suppose the metal atom has a single d electron outside of the closed shells (Ti3+ or V4+) In the free ion, the electron can be in any one of the 5 orbitals, since all are equivalent (degenerate). Recall the shapes of the d orbitals CHEM210/Chapter 5/2014/09 CHEM210/Chapter 5/2014/10 CHEM210/Chapter 5/2014/01 CHEM210/Chapter 5/2014/12 2 groups of orbitals dxy , dyz , dzx t2g dz2 , dx2- y2 eg CHEM210/Chapter 5/2014/13 Δo is the difference in energy between eg and t2g. The net energy of a t2gx egy configuration relative to the barycentre is called the ligand field stabilization energy (LFSE). LFSE = (0.4x – 0.6y)Δo HIGH- SPIN VS LOW- SPIN IN Oh COMPLEXES d4 d 1, d 2, d 3 - simple high- spin low- spin CHEM210/Chapter 5/2014/14 High-spin d 4 Low-spin d 4 t2g3 eg1 t2g4 eg0 x=3,y=1 x=4,y=0 E = (0.4x – 0.6y)Δo = 0.6 Δo E = (0.4x – 0.6y)Δo = 1.6 Δo + P CHEM210/Chapter 5/2014/15 EXAMPLE What is the LFSE for octahedral ions of the following configurations: (a) d 3 (b) high-spin d 5 SOLUTION (a) electronic configuration : t2g3eg0, x = 3, y = 0 Therefore, LFSE = (0.4x – 0.6y)Δo = [(0.4)(3) – (0.6)(0)]Δo = 1.2 Δo (b) electronic configuration : t2g3eg2, x = 3, y = 2 Therefore, LFSE = (0.4x – 0.6y)Δo = [(0.4)(3) – (0.6)(2)]Δo = 0 EXERCISE FOR THE IDLE MIND What is LFSE for both high- and low-spin d 6 configuration? CHEM210/Chapter 5/2014/16 THE SPECTROCHEMICAL SERIES The splitting of d orbitals in the CF model not only depends on the geometry of the complex, it also depends on the nature of the metal ion, the charge on this ion and the ligands that surround this ion. When the geometry and the ligands are held constant, this splitting decreases in the following order: Pt4+ > Ir3+ > Rh3+ > Co3+ > Cr3+ > Fe3+ > Fe2+ > Co2+ > Ni2+ > Mn2+ When the geometry and the metal are held constant, the splitting of the d- orbitals increases in the following order: I- < Br- < [NCS]- < Cl-< F- < OH- < H2O < NH3 < en < CN- < CO The ligand- field splitting parameter, Δo varies with the identity of the ligand. In the series of complexes [CoX(NH3)5]n+ with X = I-, Br-, Cl- H20 and NH3, the colours range from purple (for X = I-) through pink (X = Cl-) to yellow (with NH3). Ligand that give rise to high energy transition (such as CO) is referred to as a strong-field ligand; low energy transitions (such as Br-) referred to as weakfield ligand. CHEM210/Chapter 5/2014/17 MAGNETIC MEASUREMENTS Used to determine the number of unpaired spins in a complex, hence identify its ground-state configuration. Compounds are classified as diamagnetic if they are repelled by a magnetic field and paramagnetic if they are accepted by a magnetic field. The spin-only magnetic moment, μ, of a complex with total spin quantum number is given by: 𝜇 = 𝑆 𝑆 + 1 𝜇𝐵 μB = Bohr magneton CHEM210/Chapter 5/2014/18 CALCULATED SPIN-ONLY MAGNETIC MOMENTS ION N μ/μB S CALC. EXPT. Ti3+ 1 ½ 1.73 1.7-1.8 V3+ 2 1 2.83 2.7-2.9 Cr3+ 3 1½ 3.87 3.8 Mn3+ 4 2 4.90 4.8-4.9 Fe3+ 5 2½ 5.92 5.9 CHEM210/Chapter 5/2014/01 EXAMPLE The magnetic moment of a certain Co(II) complex is 4.0 μB . What is its delectron configuration? SOLUTION A Co(II) complex is d 7. Two possible configurations: t2g5eg2 (high-spin, S = 1½) with 3 unpaired electrons or t2g6eg1 (Low-spin, S = ½) with 1 unpaired electron. The spin-only magnetic moments are 3.87 μB and 1.73 μB. Therefore, the only consistent assignment is the high-spin configuration t2g5eg2. EXERCISE FOR THE IDLE MIND The magnetic moment of the complex [Mn(NCS)6]4- is 6.06 μB. What is its electron configuration? CHEM210/Chapter 5/2014/20