* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download TERATOGENITY of DRUGS
Survey
Document related concepts
Polysubstance dependence wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug interaction wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Theralizumab wikipedia , lookup
Psychopharmacology wikipedia , lookup
Transcript
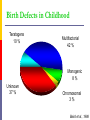
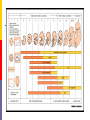
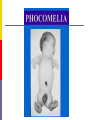
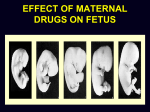
Drugs in pregnancy Jiří Slíva, MD., Ph.D. Risks of pharmacotherapy in pregnant women administration of a contraindicated drug Non-use of an indicated drug Reality SmPC/PIL: „the drug can be given only if the potential benefit for both mother and fetus outweighs the potential risks“ = ALIBISMS Categories of risk – FDA A – controlled trials in pregnant women -levothyroxin, liothironin, folic acid B – animal studie negative and controlled clinical trials not available - paracetamol C - teratogenic in animals, no clinical trials or not available in animals in women - teofylin, amlodipin D – there are known risks, but you can not substituten beta-blockers, ACEi (III. trimester) X – risks overweights the benefits oral contracetives, statins, finasterid, isotretinoin, warfarin, misoprostol, androgens Risk categories Australia (ADEC) - A, B 1-3, C, D, X A – proved as safe X – documented teratogens Germany – grades 1.–11. Teratogen is… A teratogen is an agent that can produce a permanent alteration of structure or function in an organism exposed during embyronic or fetal life. Birth Defects in Childhood Teratogens 10 % Multifactorial 42 % Monogenic 8% Unknown 37 % Chromosomal 3% Baird et al., 1988 Teratogenicity influencing factors Nature of the agent Dose Route Frequency of exposure Duration of exposure Time of exposure, i.e. gestational timing Concurrent exposures Concurrent illness Genetic susceptibility Teratogenic influence of drugs „Timing“ of teratogenic impuls („window“) before implantation – blastogenesis – low sensitivity („all or nothing“) ! 15.-55. day of gravidity – organogenesis – an increased risk (1st trimester) fetal period – usually no visible malformations but for example alteration of CNS functions Birth Defects Caused By Teratogenic Exposures Are Preventable. Public Health Concerns Prevention of known teratogenic exposures – Alcohol – Infectious diseases – Occupational exposures – Environmental exposures – Drugs abused – Medication – etc. Placenta lot of substances cause anomalies of the fetus drugs cross the placenta usually via diffusion minimal penetration is observed in highly dissociated or in lipophobic substances placenta is NOT barrier protecting the fetus from drugs administered to its mother Fetal rubeolla syndrome Fetal aminopterine syndrome Fetal hydantoine syndrome Fetal valproate syndrome Fetal warfarin syndrome Fetal alcoholic syndrome Fetal hyperpyretic syndrome Medications Over-the-counter medicines Herbals and dietary supplements Prescription drugs Frequently used by pregnant women Biologically active Taken systemically Taken in high doses Information about teratogenicity very limited Teratogenic Risk of 468 Drugs Approved 1980–2000 Undetermined None, Minimal or Unlikely Small, Moderate or High 100% 80% 60% 40% 20% 0% 0-4 5-9 10-14 15-20 Years Since FDA Approval Lo & Friedman et al., 2002 Teratogenic Risk of 468 Drugs Approved 1980-2000 11 (2.4 %) of treatments pose a “small”, “moderate” or “high” teratogenic risk On average, 6.0 ± 4.1 years after FDA approval required to recognize risk in humans 30 (6.4 %) of treatments unlikely to pose a risk in human pregnancy On average, 9.1 ± 4.5 years after FDA approval required to show safety in humans Lo & Friedman et al., 2002 Animal teratology studies are valuable but … false positives and false negatives do occur. Animal Teratology Studies: False Positives corticosteroids chlorpheniramine hydroxyzine propoxyphene Animal Teratology Studies: False Negatives ACE inhibitors: captopril, enalapril etc. Carbimazole, methimazole Misoprostol Lack of Knowledge Is a Problem 1. Exposures that really do pose a risk remain unrecognized 2. Pregnant women may not receive treatments that benefit their own health or that of the fetus 3. Labeling tends to provoke anxiety, often unnecessarily 4. Women may be advised or choose to terminate pregnancy to avoid risk Thalidomide etc. not only thalidomide, but also lenalidomide or pomalidomide are being introduced to haematooncologic praxis (MM, myelodysplastic sy etc.) inhibition of angiogenesis, hematopoesis, immunomodulation decreased synthesis of TNF-alpha and IL-6 monocytes, stimulation of T cells & NK cells Thalidomide etc. Chanan-Khan A, 2006 THALIDOMIDE Thalidomide (Contergan) – 1956 – morning nausea of pregnant women in 28 countries 100 000 kg of thalidomide, malformed more than 10.000 children – phocomelie teratogenic dose in men very low ! (0,1 mg/kg) tested several animal species (except of rabbitt) – 20–300 mg/kg only one dose is sufficient 50-100 mg in critical period (21.–36. after conception) testing of newly developed substances in pregnant women is unallowable Mechanisms of impairments often non-specific often hardly recognisible thalidomide – cca 25 of theories corticosteroids – clefts in animal but not in human direct toxicity – cytostatics, antiep. (PHE, CBZ, VAL) placentar transport – Cd multifactorial Instruments for identification of teratogenity in human pharmacology Case-reports – lithium – hearth malformations Case-control studies – stilbestrol – vaginal adenoca, ASA in I. trimester (teratogenic in animals) Cohort studies (prospective) – fluoxetine (Interventional studies) – RCT – folic acid (0.8 mg daily in prevention of neural schisis) Meta-analyses – metronidazol etc. Teratogens in the first trimester phenytoin, carbamazepine, valproate – neural tube defects (spina bifida) lithium – cardiac malformations warfarin – bone deformation, chondrodysplasy, CNS defects heparin (demineralization of bone in mother –> switch to LMWH) retinoids - def. of CNS, heart, limbs, liver oncologic drugs (fluorouracile, methotrexate, cyclophosphamide, busulfan, …) Teratogens in fetal period ACEI – renal failure, oligohydramnion thyreostatics (carbimazole, thiamazole, propylthiouracil) benzodiazepins - dependency barbiturates – dependency beta-blockers (atenolol) NSA – constriction of ductus tetracyklins – disturbances of bone mass, teeth warfarin – intracranial hemorhagies aspirin - bleeding cytostatics Conclusions think of possible pregnancy when the drug is prescribed in every women in productive age in chronicaly medicated patients (epilepsy, diabetes, hypertension) evaluate the medication so far administered not suitable to disrupt the effective pharmacotherapy without careful balancing the risk/benefit ratio …varia…. Asthma during pregnancy global increase of prevalence during last two decades the most common chronic disease during pregnancy prevalence: 3.4–12.4 % Rey, 2007 Physiologic changes durign pregnancy regarding the effects of anti-asthmatic drugs increased concentration of free cortisol in plasma (antiinflamm. eff.) increased level of progesterone (bronchodilat. eff.) increased synthesis of of potential bronchoconstrictors (PGF 2a etc.) Rey, 2007 Antiastmatics during pregnancy I. corticosteroids p.o. inactivation of prednisolone by placenta (up to 90 %) in 1st trimester higher risk of cleft palate or harelip (RR: 1.1– 1.3 %) – benefit usually outweighs the potential risk prospective, case-control study, systematic reviews => safety of the administration of: ICSs (most of studies with budesonide) SABAs theophylline cromons => not increased risk of congenital abnormalities, pre-eclampsia, preterm deliveries or small-for-date infants in most cases no need to change the medication, including dose or frequency Rey, 2007 Antiasthmatics during pregnancy II. beta-2 mimetics malformation in animals when high doses were used no malformation in human; no increase of preterm deliveries or low weight => recommendation of their combination with ICS leucotriene receptors antagonists zafirlukast – 0 teratogenity in animals; lack of studies in human => recommendation of ICS montelukast – 0 malformation or ADRs associated with pregnancy, but commonly not use Rey, 2007