* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Prevention and Treatment of VTE
Survey
Document related concepts
Transcript
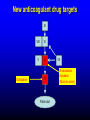
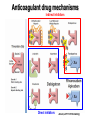
Primary prevention of VTE RATIONALE FOR THROMBOPROPHYLAXIS IN HOSPITALIZED PATIENTS - 1 High prevalence of VTE – Almost all hospitalized patients have one or more risk factors for VTE – The incidence of DVT is as high as 80% in some hospitalized patient groups – Hospital-acquired DVT and PE are usually clinically silent – It is difficult to predict which at-risk patients will develop symptomatic thromboembolic complications – Screening at-risk patients using physical examination or noninvasive testing is neither cost-effective nor effective RATIONALE FOR THROMBOPROPHYLAXIS IN HOSPITALIZED PATIENTS - 2 Adverse consequences of unprevented VTE – Symptomatic DVT and PE: postop VTE second most common medical complication – Fatal PE: PE is the most common cause of preventable hospital death – Costs of investigating symptomatic patients – Risks and costs of treating unprevented VTE – Increased future risk of recurrent VTE – Chronic postthrombotic syndrome RATIONALE FOR THROMBOPROPHYLAXIS IN HOSPITALIZED PATIENTS - 3 Efficacy of thromboprophylaxis – Thromboprophylaxis is highly efficacious at preventing DVT and proximal DVT – Thromboprophylaxis is highly effective at preventing symptomatic VTE and fatal PE – The prevention of DVT also prevents PE – Cost-effectiveness of thromboprophylaxis has repeatedly been demonstrated RISK FACTORS FOR VTE - 1 • • • • • • • Surgery Trauma (major trauma or lower-extremity injury) Immobility, lower-extremity paresis Obesity Increasing age Cancer (active or occult) Cancer therapy (hormonal, chemotherapy, angiogenesis inhibitors, radiotherapy) • Venous compression (tumor, hematoma, arterial abnormality) • Previous VTE RISK FACTORS FOR VTE • Pregnancy and the postpartum period • Estrogen-containing oral contraceptives or hormone replacement therapy • Selective estrogen receptor modulators • Erythropoiesis-stimulating agents • Acute medical illness • Inflammatory bowel disease • Nephrotic syndrome • Myeloproliferative disorders • Paroxysmal nocturnal hemoglobinuria • Central venous catheterization • Inherited or acquired thrombophilia • Family history of VTE THROMBOPHILIA • Inherited – Antithrombin deficiency – Protein C deficiency – Protein S deficiency – Factor V Leiden (heterozygous or homozygous) – Prothrombin G20210A gene mutation • Acquired – Antiphospholipid syndrome Highest risk: Antithrombin deficiency, homozygous Factor V Leiden or compound heterozygotes, antiphospholipid syndrome Predictive value of family history as good as that of lab testing RISK OF DVT IN HOSPITALIZED PATIENTS NOT RECEIVING PROPHYLAXIS Most thrombotic events occur after hospital discharge DRUG REGIMENS TO PREVENT VTE • Low dose unfractionated heparin (5000 U q 8-12h) • Low molecular weight heparin (dalteparin 2500 U q 12-24h; enoxaparin 30 mg q 12h or 40 mg daily) • Fondaparinux (2.5 mg sq once daily) • Warfarin: Adjust to target INR 2-3 • Low-dose ASA • New oral agents: dabigatran, rivaroxaban, apixaban THROMBOPROPHYLACTIC DRUGS Agent Advantages Disadvantages Heparin Cost HIT risk Shorter half-life LMWH Lower risk of HIT Once daily dosing option Cost High blood levels in renal failure Warfarin Oral administration Cost No HIT risk Variable dose-response Delayed onset of effect Need for monitoring Fondaparinux Efficacy? (vs LMWH) Once daily dosing Minimal HIT risk Cost Higher bleeding risk? High blood levels in renal failure Relative efficacy of various thromboprophylactic regimens following THR: meta-analysis Treatment All DVT (%) Prox DVT (%) Bleeding (%) None 47 23 0.3 Aspirin 36 16 0.4 UFH 24 14 2.6 LMWH 17 6 1.8 Stockings 18 13 0 Warfarin 24 5 1.3 JAMA 1994;271:22 Unfractionated heparin in general surgery • Meta-analysis of 46 RCTs comparing UFH and placebo or no treatment • UFH reduced DVT rate from 22% to 9% • Reduced symptomatic PE rate from 2.0% to 1.3% • Reduced fatal PE rate from 0.8% to 0.3% • Reduced all cause mortality from 4.2% to 3.2% (one less death per 97 patients treated) • Increased bleeding rate from 3.8% to 5.9% (most bleeds minor) N Engl J Med 1988; 318:1162 LMWH in surgery • General surgery: – LMWH reduces risk of asymptomatic DVT and symptomatic VTE by over 70% vs no treatment – Roughly equivalent to UFH in terms of efficacy and safety • LMWH appears superior to UFH in high-risk orthopedic surgery • No study has shown clear superiority of one form of LMWH over another 2008 ACCP guidelines FONDAPARINUX • Selective Xa inhibitor (does not inhibit thrombin) • Long half-life (once daily dosing), no antidote • Equivalent or slightly superior to LMWH for prevention of postoperative VTE – Slightly higher bleeding risk FONDAPARINUX VS ENOXAPARIN IN ORTHOPEDIC SURGERY Pooled results from four pivotal trials Outcome Fondaparinux Enoxaparin Odds Ratio (95% CI) All VTE 6.8% 13.7% 0.45 (0.37-0.54) Proximal DVT 1.3% 2.9% 0.43 (0.27-0.64) Major Bleed 2.7% 1.7% 1.54 (1.11-2.16) Lancet 2002;359:1710 MECHANICAL THROMBOPROPHYLAXIS Graded compression stockings – Knee- or thigh-high Intermittent pneumatic compression Venous foot pump Mechanical thromboprophylaxis • Advantages – No bleeding risk – Demonstrated efficacy (but limited evidence) – Enhance efficacy of anticoagulant prophylaxis – Reduce leg swelling • Disadvantages – Less well-studied than anticoagulants – Less well-standardized – Not all devices have been evaluated in trials Less effective in high-risk groups Less effective in preventing proximal DVT Not shown to prevent PE or death – Compliance issues Thromboprophylaxis in acutely ill medical patients 2012 ACCP recommendations • Patients with increased VTE risk: – LMWH, low-dose UFH or fondaparinux • Patients with low VTE risk: – No prophylaxis • Patients who are bleeding or at high risk for bleeding: – No anticoagulant prophylaxis – Mechanical prophylaxis if VTE risk high Chest 2012;141:7S-47S VTE Risk in Surgery: Rogers Score J Am Coll Surg 2007;204:1211 VTE Risk in Surgery: Caprini Score Dis Mon 2005; 51:70 VTE Risk in Surgery • Very low risk (< 0.5%): – Rogers score < 7 – Caprini score 0 • Low risk (~ 1.5%) – Rogers score 7-10 – Caprini score 1-2 • Moderate risk (~ 3%) – Rogers score > 10 – Caprini score 3-4 • High risk (≥ 6%) – Caprini score ≥ 5 Thromboprophylaxis in non-orthopedic surgical patients 2012 ACCP recommendations • Very low VTE risk: – No prophylaxis, early ambulation • Low VTE risk: – Mechanical prophylaxis (IPC preferred) • Moderate VTE risk, not high bleeding risk: – LMWH, low dose UFH, or mechanical prophylaxis • Moderate VTE risk, high bleeding risk – Mechanical prophylaxis (IPC) • High VTE risk, not high bleeding risk: – LMWH or low dose UFH, plus mechanical prophylaxis Chest 2012;141:7S-47S Thromboprophylaxis in non-orthopedic surgical patients 2012 ACCP recommendations (2) • Cancer surgery, not high bleed risk: – Extended duration LMWH (4 weeks) • High VTE risk, high bleed risk: – Mechanical prophylaxis (IPC), pharmacologic prophylaxis once bleed risk diminishes • High VTE risk, LMWH and UFH contraindicated, not high bleed risk: – Low dose ASA, fondaparinux, or mechanical • IVC filter should NOT be used for primary VTE prevention Chest 2012;141:7S-47S Thromboprophylaxis in orthopedic surgical patients 2012 ACCP recommendations • Total hip or knee arthroplasty: – LMWH (preferred), low-dose UFH, fondaparinux, adjusteddose warfarin, apixaban, dabigatran, rivaroxaban, aspirin (controversial), plus IPC • Hip fracture surgery: – LMWH (preferred), low-dose UFH, fondaparinux, adjusteddose warfarin, aspirin (controversial), plus IPC • LMWH should be given at least 12 hours pre-op or 12 hours post-op rather than closer to the time of surgery • Prophylaxis should be continued for a minimum of 10-14 days • IPC only if high bleeding risk; IVC filter if there is contraindication to IPC Chest 2012;141:7S-47S Prolonged Thromboprophylaxis Decreases VTE Risk in Major Orthopedic Surgery • Meta-analysis of 8 randomized controlled trials • Prolonged prophylaxis (≥ 21 days, vs 7-10 days) decreased VTE risk: – 86% reduction in risk of PE – 74% reduction in risk of symptomatic DVT – 71% reduction in risk of proximal DVT • 2.4-fold increase in risk of minor bleeding with prolonged prophylaxis Ann Intern Med 2012;156:720 KNEE ARTHROSCOPY • Symptomatic DVT rate < 1% without prophylaxis Routine thromboprophylaxis not recommended Prophylaxis (eg, LMWH) recommended for patients with prior hx of VTE 2012 ACCP guidelines SPINAL OR EPIDURAL ANESTHESIA • Reports of perispinal hematomas in patients receiving LMWH – Exact prevalence unknown – Few reports with low dose UFH as well • Risk factors: – coagulopathy – anatomic spine abnormalities – difficult insertion/repeated attempts – higher doses of anticoagulant – continuous epidural catheter – older age SPINAL OR EPIDURAL ANESTHESIA RECOMMENDATIONS • Avoid in patients with known coagulopathy • D/C clopidogrel (Plavix) at least 5 days before – ASA safer? • Needle insertion and epidural catheter removal at least 8 hours after last dose of LMWH if twice daily, or 18 h after last dose if once daily • Wait at least 2h before restarting LMWH, longer if CSF bloody • Do not use continuous epidural anesthesia for more than 2 days if pt taking warfarin; INR should be < 1.5 when catheter removed • Fondaparinux not recommended (long half-life, little data) • Monitor for signs of cord compression 2008 ACCP guidelines Variables That Should Be Considered In Choice Of Thromboprophylaxis 2012 ACCP recommendations “In choosing the specific anticoagulant drug to be used for pharmacoprophylaxis, choices should be based on patient preference, compliance, and ease of administration (eg, daily vs bid vs tid dosing), as well as on local factors affecting acquisition costs (eg, prices of various pharmacologic agents in individual hospital formularies)” Chest 2012;141:7S-47S Treatment of acute VTE Principles of VTE Treatment • Adequate treatment of VTE requires administration of a rapidacting anticoagulant • This drug should be given in doses sufficient to achieve a systemic anticoagulant effect, eg: – UFH: 70-80 U/kg loading dose, 15-18 U/kg/h infusion with aPTT monitoring – Enoxaparin: 1 mg/kg sq twice daily No routine monitoring – Dalteparin: 100 U/kg sq twice daily – Fondaparinux: 7.5 mg sq daily • Initial treatment should be given for a minimum of 5 days • Failure to administer sufficient doses of a rapid-acting anticoagulant may increase risk of recurrent VTE for up to three months Heparin is superior to a vitamin K antagonist for initial treatment of acute DVT 14 Heparin + acenocoumarol Cumulative failures 12 Acenocoumarol alone 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Weeks Brandjes et al, NEJM 1992;327:1485 HEPARIN SHOULD BE DOSED ACCORDING TO BODY WEIGHT A randomized, controlled trial in 115 patients with thromboembolism or unstable angina (Ann Intern Med 1993;119:874) Weight-based starting dose: 80 U/kg bolus, 18 U/kg/hr Standard starting dose: 5000 U bolus, 1000 U/hr Outcome Standard Weight-based P value dose dose First aPTT > 1.5 x control, % 32 86 <0.001 aPTT > 1.5 x control within 24 hours, % 77 97 0.002 Minor bleeding, % 3.8 3.2 NS Major bleeding, % 1.9 0 NS Recurrent DVT/PE, % 25 5 0.02 HEPARIN "RESISTANCE" Causes and solutions • • • • • Inadequate dose (large patient) Solution: weight-based dosing aPTT prolongation less than expected despite therapeutic heparin level (base aPTT short) Solution: monitor heparin level (anti-Xa activity) Heparin neutralized by PF4 released during clot formation Solution: LMWH/fondaparinux Low plasma antithrombin level (very rarely a cause) Solution: antithrombin concentrate or FFP infusion Heparin antibodies (may cause thrombocytopenia and thrombosis) Solution: direct thrombin inhibitor (lepirudin, etc) or fondaparinux LOW MOLECULAR WEIGHT HEPARIN Advantages over standard heparin • Better bioavailability • Longer half-life allows once or twice daily dosing – Facilitates outpatient treatment • Most patients do not need monitoring • Less likely than to cause HIT • Less bone mineral loss, lower fracture risk • Disadvantages – Accumulates in renal failure – Not neutralized as well by protamine ENOXAPARIN LEVEL VS CREATININE CLEARANCE J Clin Pharmacol 2003;43:586-590 Patients treated with enoxaparin 1 mg/kg q12h Conclusion: monitoring warranted when CrCl < 30 STANDARD VS LMW HEPARIN FOR TREATMENT OF DVT meta-analysis of 10 published trials Outcome % Risk reduction with LMWH 95% CI Symptomatic thromboembolism 53 18-73 Clinically important bleeding 68 31-85 Mortality 47 10-69 Arch Intern Med 1995;155:601-7 LMWH vs UFH • Low molecular weight heparin is at least as effective as unfractionated heparin in the treatment of acute VTE • Low molecular weight heparin has significant practical advantages over unfractionated heparin • 2012 ACCP Guidelines prefer once-daily LMWH or fondaparinux over UFH for initial treatment of acute VTE Warfarin for prevention of recurrent VTE • Takes minimum of 4-5 days to establish anticoagulant effect • INR does not reflect anticoagulant effect for first 23 days • Target INR 2-3 • Utility of “loading dose” questionable It takes at least 4-5 days for warfarin to achieve an adequate anticoagulant effect Clotting factor levels after starting warfarin New Oral Anticoagulants New anticoagulant drug targets XI Dabigatran VIII IX V X II Fibrin clot VII Rivaroxaban Apixaban (More to come) New oral anticoagulants • Dabigatran (Pradaxa®) – thrombin inhibitor – FDA approval 2010: stroke prevention in non-valvular Afib; approved 2014 for VTE treatment • Rivaroxaban (Xarelto®) – Xa inhibitor – FDA approval 2010/11: postop VTE prophylaxis, stroke prevention in Afib, treatment of VTE • Apixaban (Eliquis®) – Xa inhibitor – FDA approval 2012: stroke prevention in Afib; approved 2014 for VTE prophylaxis after major orthopedic surgery – FDA approval for VTE treatment 2014 • Edoxaban – Xa inhibitor – Not yet FDA approved Anticoagulant drug mechanisms Indirect inhibitors Direct inhibitors Ansell, 2011 HTRS meeting Pharmacology of oral anticoagulant drugs Warfarin New agents Bioavailability 99% 6-80% (some active drug in large bowel) Tmax 72-96 hours 2-4 hours Half-life 40 hours 5-17 hours Metabolism Cytochrome P450 Biliary/Renal Drug Interactions Many Not so many Food Interactions Yes No Genetic Variation Major effects Minor effects (?) Monitoring PT/INR None Reversal Vit K/PCC/FFP PCC? Dialysis? Cost per month of oral anticoagulants • Rivaroxaban (20 mg/day) : $290 • Dabigatran (150 mg bid): $290 • Apixaban (5 mg bid): $147 • Warfarin (7.5 mg/day): $31 Source: UWHC Pharmacy Dabigatran • Dose – Stroke prevention in A fib: 110-150 mg bid • 110 mg dose not available in US • For patients with CrCl 15-30: 75 mg bid • Not recommended for CrCl < 15 or dialysis dependent – Postop VTE prophylaxis*: 150-220 mg once daily – VTE treatment/prevention of recurrent VTE: 150 mg bid • Less than 10% absorbed; relatively high rate of GI side effects • Crosses the placenta – do not use during pregnancy • Drug may degrade over time after exposure to air – must be kept in original packaging Unused tablets should be discarded after 90 days * Not FDA-approved indication Rivaroxaban • Dose: – Stroke prevention in Afib: 15-20 mg once daily – Post op VTE prophylaxis: 10 mg once daily – Acute VTE treatment: 15 mg twice daily – Secondary prevention of VTE: 20 mg once daily – Acute coronary syndrome*: 2.5-5 mg twice daily • Use with caution in moderate renal impairment (CrCL 3049); 15 mg/day dose recommended – Avoid use if CrCl < 30 (not dialyzable) • Avoid use in severe liver disease *Not FDA-approved indication Apixaban • Dose: – Stroke prevention in Afib: 5 mg bid • 2.5 mg bid if age >80, weight < 60 kg, or serum creatinine > 1.5 – Post op VTE prophylaxis: 2.5 mg bid – Treatment of acute VTE*: 10 mg bid – Secondary prevention of VTE*: 2.5 - 5 mg bid • Avoid use in severe liver disease (75% biliary excretion) *Not FDA-approved indication RESULTS OF AF TRIALS WITH NEW ORAL AGENTS • Main result: New agents at least as effective as warfarin, can be given without routine monitoring • Other findings: – Reduction in intracranial bleeding – Slightly higher MI rates – Higher rates of GI bleeding (active drug in lower intestine) – Extracranial bleeding risk higher in older patients Dabigatran vs warfarin for acute VTE The RE-COVER trial Treatment VTE recurrence Major bleeding Any bleeding Dabigatran 2.4% 1.6% 16.1% Warfarin 2.1% 1.9% 21.9% Conclusion: A fixed dose of dabigatran is as effective and safe as warfarin for treatment of acute venous thromboembolism NEJM 2009; 361: 2342 Rivaroxaban for acute VTE The EINSTEIN-DVT trial Treatment Recurrent VTE Bleeding Rivaroxaban 2.1% 8.1% Standard treatment 3.0% 8.1% Conclusion: rivaroxaban is as effective and safe as standard treatment for acute VTE NEJM 2010; 363: 2499 Rivaroxaban for Pulmonary Embolism The EINSTEIN-PE trial Treatment Recurrent VTE Bleeding Major Bleeding Rivaroxaban 2.1% 10.3% 1.1% Standard treatment 1.8% 11.4% 2.2% Conclusion: rivaroxaban as effective as standard treatment for initial and extended treatment of pulmonary embolism, may be safer NEJM 2012; 366: 1287 Dabigatran vs enoxaparin prophylaxis after total knee or hip arthroplasty RE-MODEL RE-NOVATE J Thromb Haemost 2007;5:2178 Lancet 2007;370:949 Surgery knee hip # pts 2076 3494 Drug doses Dab: 150 or 220 qd Enox: 40 mg qd Dab: 150 or 220 qd Enox: 40 mg qd Duration (d) 6-10 28-35 VTE or death (%) D150: 40.5 D220: 36.4 E: 37.7 D150: 8.6 D220: 6.0 E: 6.7 Major Bleeding (%) D150: 1.5 D220: 1.3 E: 1.3 D150: 1.3 D220: 2.0 E: 1.6 Both trials showed dabigatran (either dose) had similar efficacy and safety compared to enoxaparin Rivaroxaban vs enoxaparin prophylaxis after total knee or hip arthroplasty: the RECORD trials RECORD 1 RECORD 2 RECORD 3 RECORD 4 NEJM 2008;358:2775 Lancet 2008;372:31 NEJM 2008;358:2776 Lancet 2009;373:1673 Surgery hip hip knee knee # pts 4541 2509 2531 3148 Duration (d) 35 Riv: 31-35 Enox: 10-14 14 11-15 VTE or death (%) R: 1.1 E: 3.7 R: 2.0 E: 9.3 R: 9.6 E: 18.9 R: 6.9 E: 10.1 Bleeding (%) R: 0.3 R: 6.6 R: 0.6 R: 0.7 E: 0.1 E: 5.5 E: 0.5 E: 0.3 (major bleed) 10 (anymg/d bleed)vs enoxaparin (major bleed) 40 (major bleed) rivaroxaban mg/d All trials with All had mandatory venography All showed rivaroxaban had superior efficacy vs enoxaparin with similar safety Apixaban vs enoxaparin prophylaxis after total knee or hip arthroplasty: the ADVANCE trials ADVANCE 1 ADVANCE 2 ADVANCE 3 NEJM 2009;361:594 Lancet 2010;375:807 NEJM 2010;363:2487 Surgery knee knee hip # pts 3195 3057 5407 # evaluable for efficacy 2287 1973 3866 Duration (d) 10-14 10-14 35 VTE or death (%) A: 9.0 E: 8.8 A: 15 E: 24 A: 1.4 E: 3.9 Bleeding (%) A: 2.9 E: 4.3 A: 4 E: 5 A: 4.8 E: 5.0 Meta-analysis of data from ADVANCE-1 and ADVANCE-2 shows that apixaban is non-inferior to enoxaparin with respect to efficacy, and has a “considerable” safety advantage (Huang et al, Thromb Haemost 2011;105: 245) Effect of dabigatran on PT and aPTT Trough levels: 32-225 ng/ml Peak levels: 64-443 ng/ml • Neither assay is adequately sensitive in the range of concentrations expected in patients on chronic oral therapy • Sensitivity to drug level is reagent-dependent, will vary among laboratories Thromb Haemost 2010;103:1116 • No specific antidote for any of the new OACs • Activated charcoal will reduce drug absorption if administered within a few hours of ingestion • Rivaroxaban & apixaban effect may be reversed by giving prothrombin complex concentrate (PCC) • Dabigatran is dialyzable • Case reports suggest that recombinant factor VIIa (NovoSeven™) is ineffective vs dabigatran (Thromb Haemost 2012;108:585) When to stop drug before surgery Dabigatran CrCl, mL/min Approx halflife, h Standard risk surgery High risk surgery >80 13 24 h 2 days 50-80 15 24h 2 days 30-50 18 2 days 4 days <30 27 4 days 6 days Rivaroxaban CrCl, mL/min Approx halflife, h Standard risk surgery High risk surgery >30 12 24 h 2 days <30 ? 2 days 4 days